Guanidyl functionalized ionic liquid as well as preparation method and application thereof
An ionic liquid and functional technology, which is applied in the field of sulfur dioxide capture and conversion, can solve the problems of hindering SO2 mass transfer and absorption diffusion, low absorption capacity, high viscosity of ionic liquid, etc., to achieve good absorption and capture, high absorption The effect that capacity, absorption capacity is high
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 5.76g (0.05mol) of 1,1,3,3-tetramethylguanidine to 50ml of absolute ethanol, dissolve in a 250mL flask, slowly drop into 4.50g (0.05mol) of methoxyacetic acid under ice-water bath, stir , After reacting for 24h, ethanol and water were removed by rotary evaporation, and the residue was crude product. Then vacuum-dry at 323.2K for at least 48 hours to obtain the ionic liquid 1,1,3,3-tetramethylguanidine methoxyacetate [TMG][MOAc].
[0031] Its chemical structural formula is:
[0032]
[0033] The prepared ionic liquid has a viscosity of 295.2cP and a density of 1.09623g.cm at 30°C. -3 .
[0034] Characterization results: [TMG][MOAc]: 1 H NMR (300MHz, CDCl 3 )δ3.83-3.82(d,2H),3.69-3.66(dddd,J=12.2,7.0,5.1,2.3Hz,1H),3.37–3.36(m,3H),2.98–2.97(m,12H), 1.24-1.17(m,1H). 13 C NMR (75MHz, CDCl 3 )δ175.2, 162.2, 72.6, 58.2, 57.1, 39.5, 18.2. HRMS (EI + )Calcdfor[C 5 h 14 N 3 ](M + ): 116.11822, found 116.11833; HRMS (EI-) Calcd for [C 3 h 5 o 3 ](M - ):89.02...
Embodiment 2
[0036] With a method similar to Example 1, 5.76g (0.05mol) 1,1,3,3-tetramethylguanidine was added to 50ml of absolute ethanol, dissolved in a 250mL flask, and slowly dripped into 5.21g ( 0.05mol) ethoxyacetic acid, stirred, reacted for 24h, and removed ethanol and water by rotary evaporation, and the residue was crude product. Then vacuum-dry at 323.2K for at least 48 hours to obtain the ionic liquid 1,1,3,3-tetramethylguanidine ethoxy acetate [TMG][EOAc].
[0037] Its chemical structural formula is:
[0038]
[0039] The prepared ionic liquid has a viscosity of 184.4cP and a density of 1.06359g.cm when tested at 30°C -3 .
[0040] Characterization results: 1 H NMR (300MHz, CDCl 3 )δ3.88(d, J=0.7Hz, 2H), 3.72-3.70(qd, J=7.0,0.7Hz, 1H), 3.58-3.51(qd, J=7.1,0.6Hz, 2H), 2.98(s ,12H),1.25-1.18(tdd,J=7.1,3.8,0.6Hz,4H). 13 C NMR (75MHz, CDCl 3 )δ175.8, 162.4, 77.6, 77.2, 76.7, 70.8, 65.9, 57.5, 39.6, 18.3, 15.1. HRMS (EI + ) Calcd for [C 5 h 14 N 3 ](M + ):116.11822, ...
Embodiment 3
[0042] Weigh 1 g of 1,1,3,3-tetramethylguanidine methoxyacetate [TMG][MOAc] synthesized in Example 1 and place it in the absorption cell, vacuumize the absorption cell, and keep the temperature at 30°C , to pass SO into the absorption pool 2 Absorption, when the pressure in the absorption pool is maintained for 30 minutes to balance, it shows that the ionic liquid absorbs SO 2 Has reached saturation. Experimentally, when SO 2 When the partial pressure is 100kPa, SO 2 The absorption capacity is 2.00mol SO 2 / mol ILs(9.76mol SO 2 / kg ILs).
[0043] [TMG][MOAc] absorbs SO 2 The mechanism is as follows:
[0044]
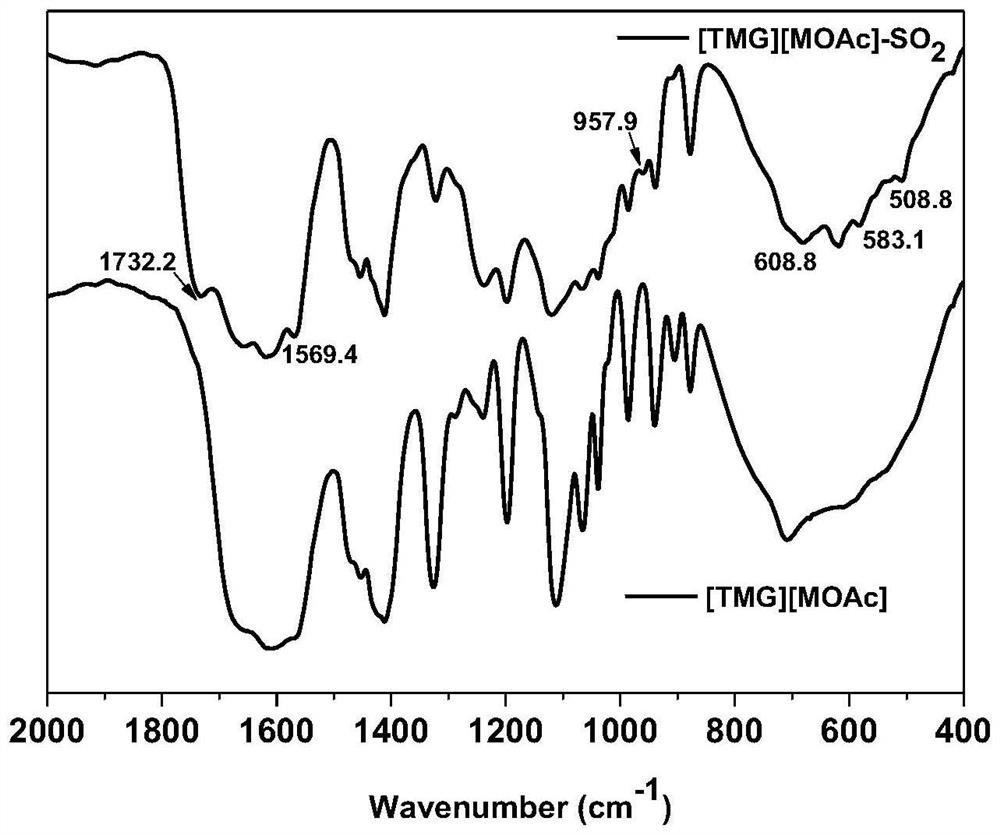
[0045] see figure 1 , [TMG][MOAc] absorbs SO 2 Infrared spectra before and after. Contrast absorption of SO 2 Before and after spectra, at 583.1 and 508.8cm -1 A new peak appeared at , which is due to the shear bending vibration of S=O, at 957.9cm -1 A new peak appears at , which is the asymmetric stretching vibration of S=O. Absorption of SO in [TMG][MO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com