Method for preparing methyl methoxyacetate by industrial aqueous raw material methylal
A technology for the preparation of methyl methoxyacetate and methylal, applied in the directions of carbon monoxide or formate reaction preparation, organic chemistry, etc., can solve problems such as impossibility, and achieve low pollution, convenient source of raw materials, and few by-products. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
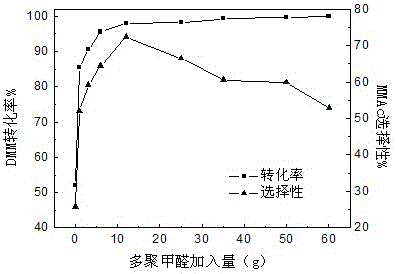
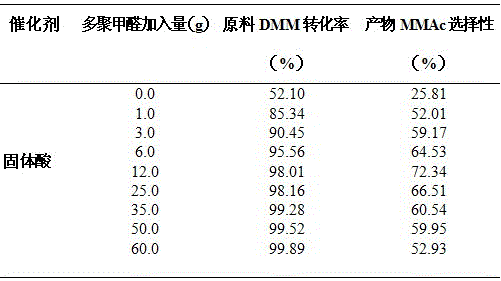
[0053] Weigh 2 L of water-containing 2% methylal (DMM), 100 g of solid acid catalyst, and a certain mass of paraformaldehyde (0.0, 1.0, 3.0, 6.0, 12.0, 25.0, 35.0, 50.0, 60.0 g) into the reactor middle. Then pass in 1.0 MPa carbon monoxide gas, if there is no leak, after emptying the gas in the kettle, repeat the above operation twice (replace the air in the reaction kettle). Introduce a certain amount of gas (6.0MPa) again to test for leaks, and let it stand for 15 minutes. If the pressure indicator does not drop, it means that there is no air leakage in the device. Then empty the gas in the kettle and fill the kettle with 5 MPa CO gas again. , heated up, the stirring speed of the reactor was 500 rpm, the reaction pressure was 5 MPa, the reaction temperature was 110°C, and the reaction time was 6 h. The reaction results are shown in Table 1.
[0054] Table 1 Effects of different paraformaldehyde additions on the carbonylation reaction of 2% DMM in water
[0055]
[0056]...
Embodiment 2
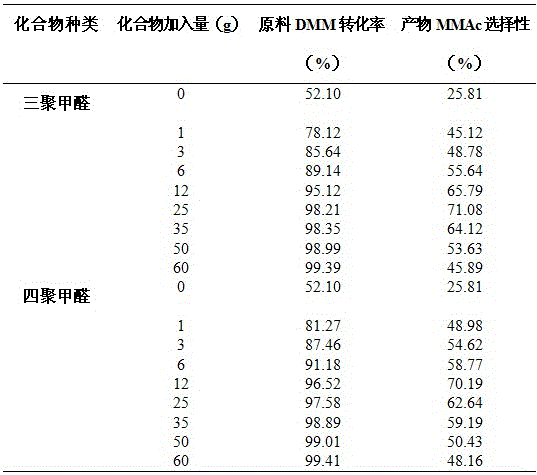
[0058] Weigh 2 L of 10% methylal (DMM) containing water, 100 g of solid acid catalyst, and a certain mass of paraformaldehyde (0.0, 2.0, 6.0, 12.0, 25.0, 50.0, 70.0, 100.0, 120.0 g) in a reaction kettle. Then pass in 1.0 MPa carbon monoxide gas, if there is no leak, after emptying the gas in the kettle, repeat the above operation twice (replace the air in the reaction kettle). Introduce a certain amount of gas (6.0 MPa) again to test for leaks, and let it stand for 15 minutes. If the pressure indicator does not drop, it means that there is no air leakage in the device. Then empty the gas in the kettle and fill the kettle with 5 MPa CO gas again. , heated up, the stirring speed of the reactor was 500 rpm, the reaction pressure was 5 MPa, the reaction temperature was 110 °C, and the reaction time was 6 h. The reaction results are shown in Table 2.
[0059] Table 2 Effects of different amounts of paraformaldehyde added on the carbonylation reaction of 10% DMM in water
[0060] ...
Embodiment 3
[0064] Add 100 g of solid acid catalyst into the reaction kettle, 2 L of reaction raw material DMM containing 2% water, and 12 g of paraformaldehyde. Then pass in 1.0 MPa carbon monoxide gas, if there is no leak, after emptying the gas in the kettle, repeat the above operation twice (replace the air in the reaction kettle). Introduce a certain amount of gas (6.0 MPa) again for leak detection, and let it stand for 15 minutes. If the pressure indicator does not drop, it means that the device has no air leakage. Then empty the gas in the kettle and inject 5 MPa pure CO gas. The reaction time 6 h, the reaction temperatures were 90, 100, 110, 130, 140°C, respectively.
[0065] Table 3 Effect of different reaction temperatures on the carbonylation reaction of DMM
[0066]
[0067] According to Table 3, it can be seen that with the increase of temperature, the conversion rate of raw material DMM increases continuously. When the reaction temperature is 140 °C, the conversion rate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com