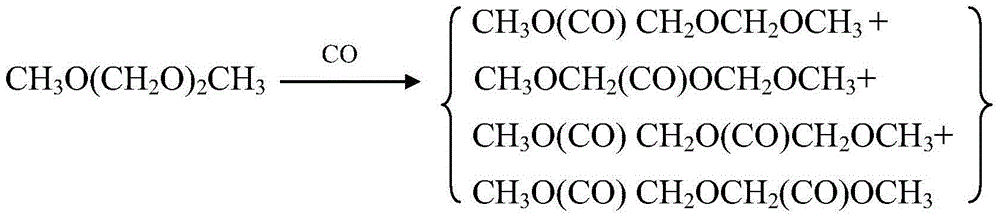
Method of improving performance of methylal carbonylation reaction catalyst
A carbonylation reaction and methylal technology, which is applied in the field of improving the performance of methylal carbonylation reaction catalysts, can solve the problems of many process units and high purity requirements of industrial gases, and achieve easy separation, high activity and selectivity, and long life. Enhanced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
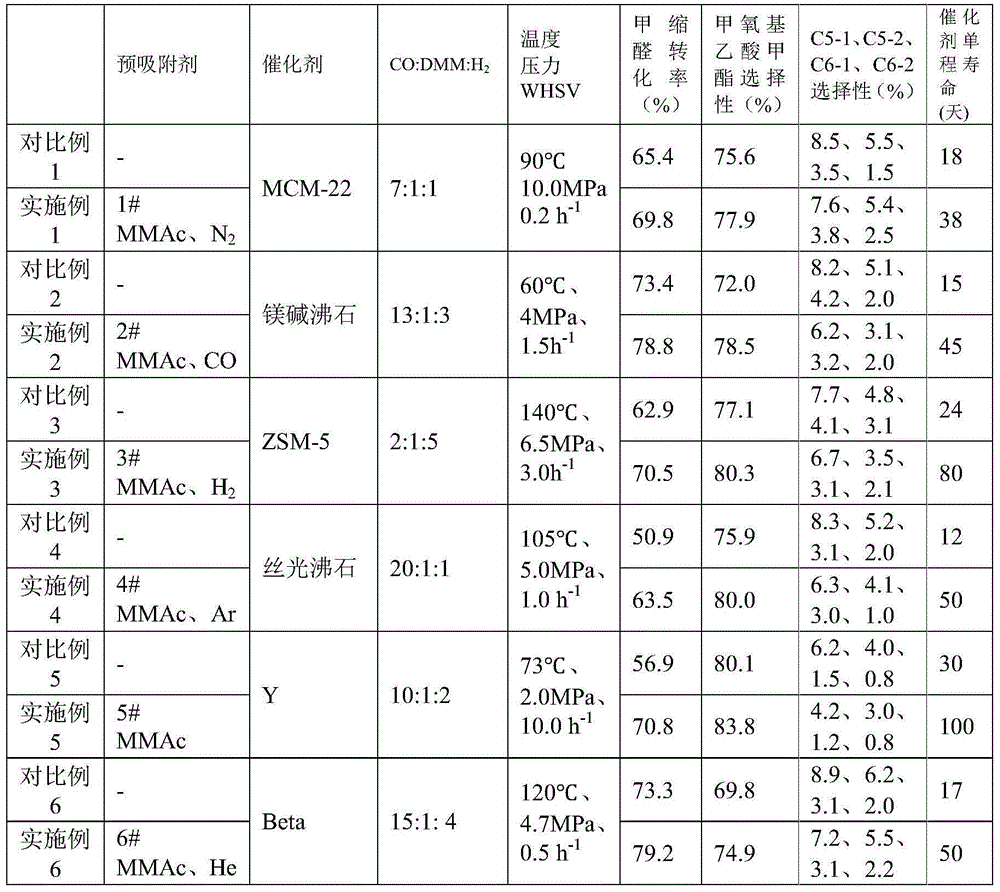
[0059] When the catalyst in Comparative Example 1 was activated by nitrogen and dropped to the reaction temperature, the pre-adsorbent 1# was methyl methoxyacetate and nitrogen. Pre-adsorption conditions: the flow rate of methyl methoxyacetate is 0.04ml / min (standard state volume flow rate, the same below), the nitrogen flow rate is 100ml / min, it is saturated after 90 minutes of adsorption, then stop feeding pre-adsorbent 1#, and start at the same time The reaction raw materials were introduced, and the remaining experimental steps and reaction conditions were consistent with Comparative Example 1. The reaction results are shown in Table 1.
Embodiment 2
[0063] When the catalyst in Comparative Example 2 was activated by nitrogen and dropped to the reaction temperature, the pre-adsorbent 2# was methyl methoxyacetate and carbon monoxide. Pre-adsorption conditions: the flow rate of methyl methoxyacetate is 0.08ml / min, the flow rate of carbon monoxide is 150ml / min, saturated after 60 minutes of adsorption, then stop feeding pre-adsorbent 2#, and start feeding the reaction raw materials at the same time, the rest of the experimental steps are the same as The reaction conditions were consistent with Comparative Example 2, and the reaction results are shown in Table 1.
Embodiment 3
[0067] When the catalyst in Comparative Example 3 was activated by nitrogen and dropped to the reaction temperature, pre-adsorbent 3# methyl methoxyacetate and hydrogen. Pre-adsorption conditions: the flow rate of methyl methoxyacetate is 0.06ml / min, the flow rate of hydrogen gas is 120ml / min, it is saturated after 100 minutes of adsorption, then stop feeding pre-adsorbent 3#, and start feeding the reaction raw materials at the same time, the rest of the experimental steps are the same as The reaction conditions were consistent with Comparative Example 3, and the reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com