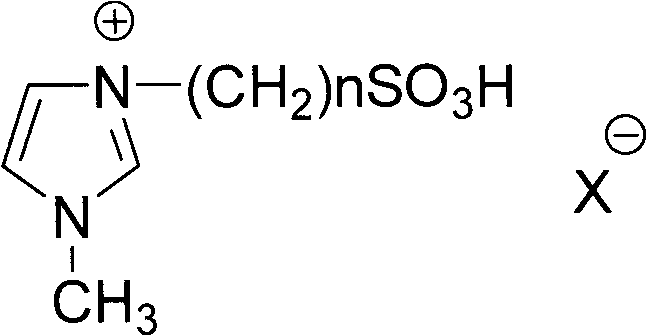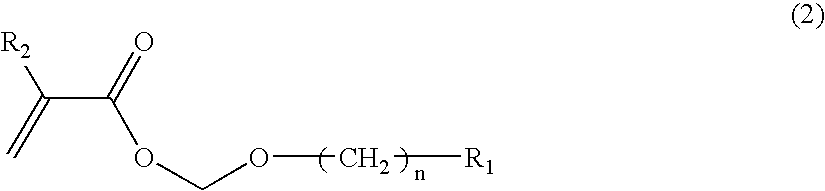Patents
Literature
490 results about "Acid strength" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
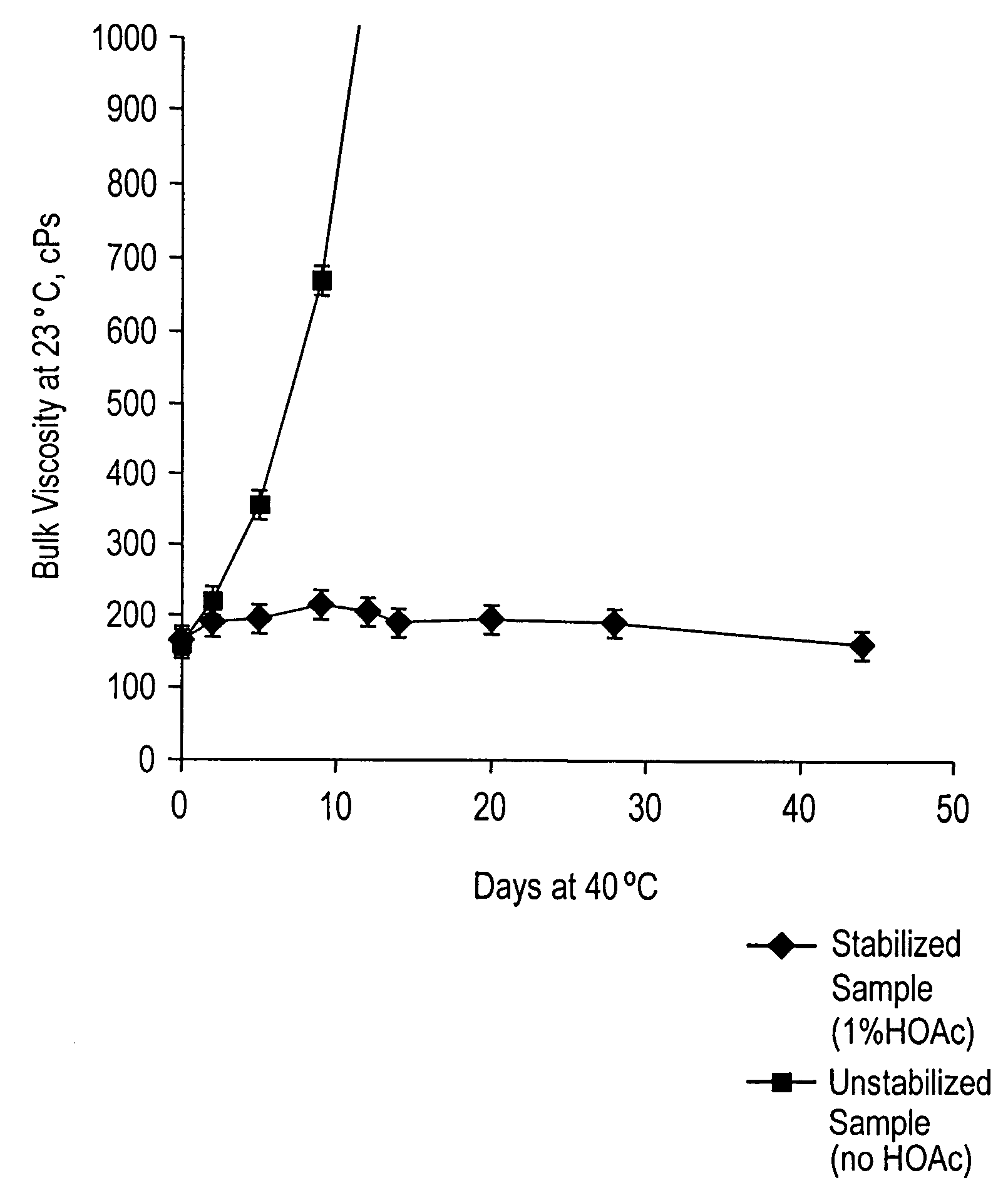
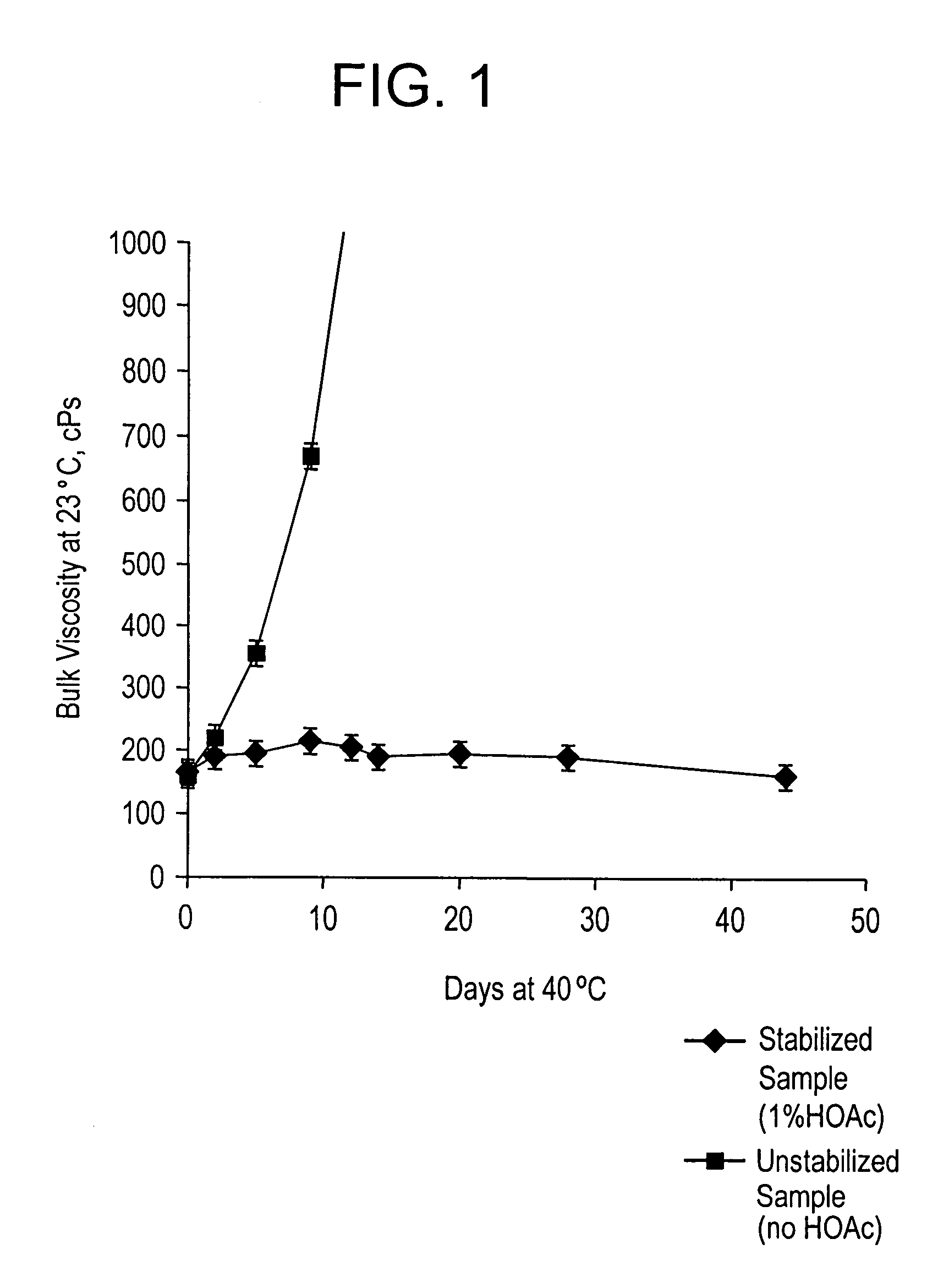
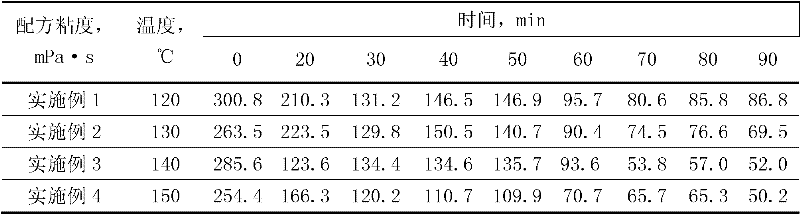
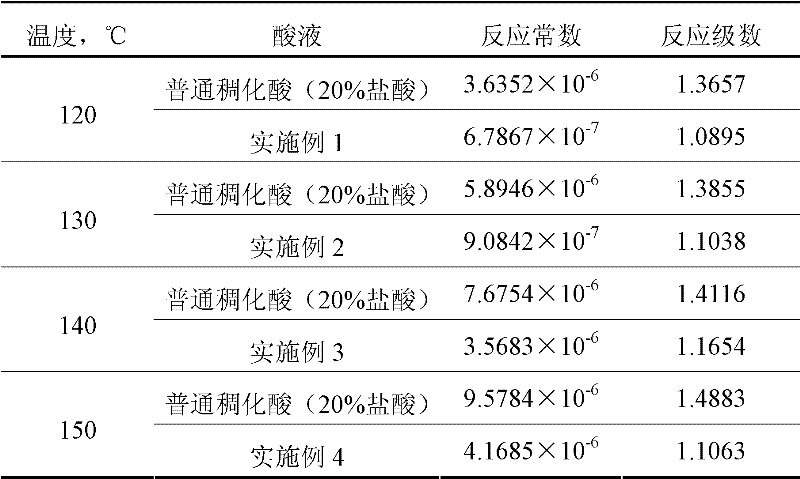
Acid strength refers to the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H⁺, and an anion, A⁻. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions.
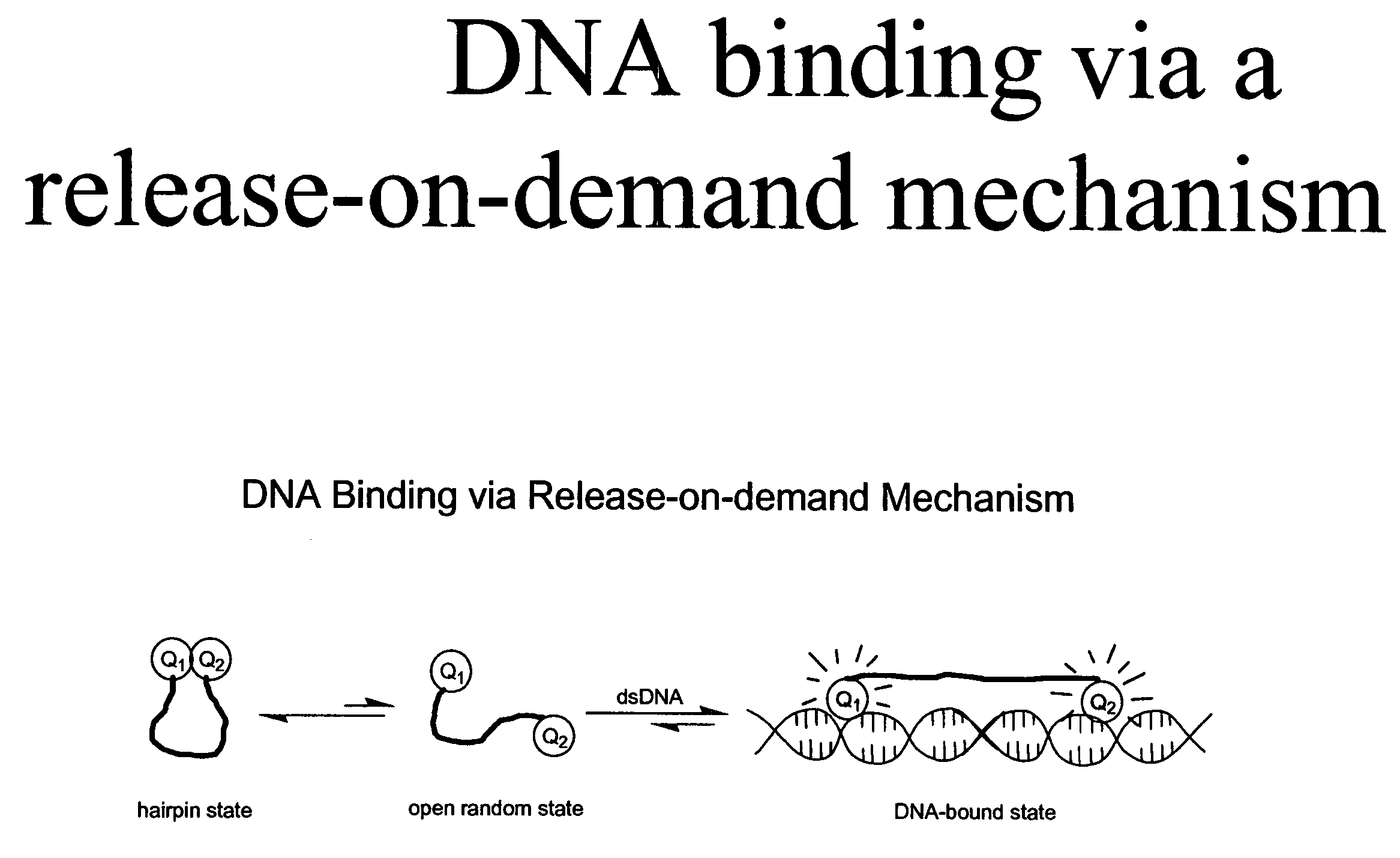
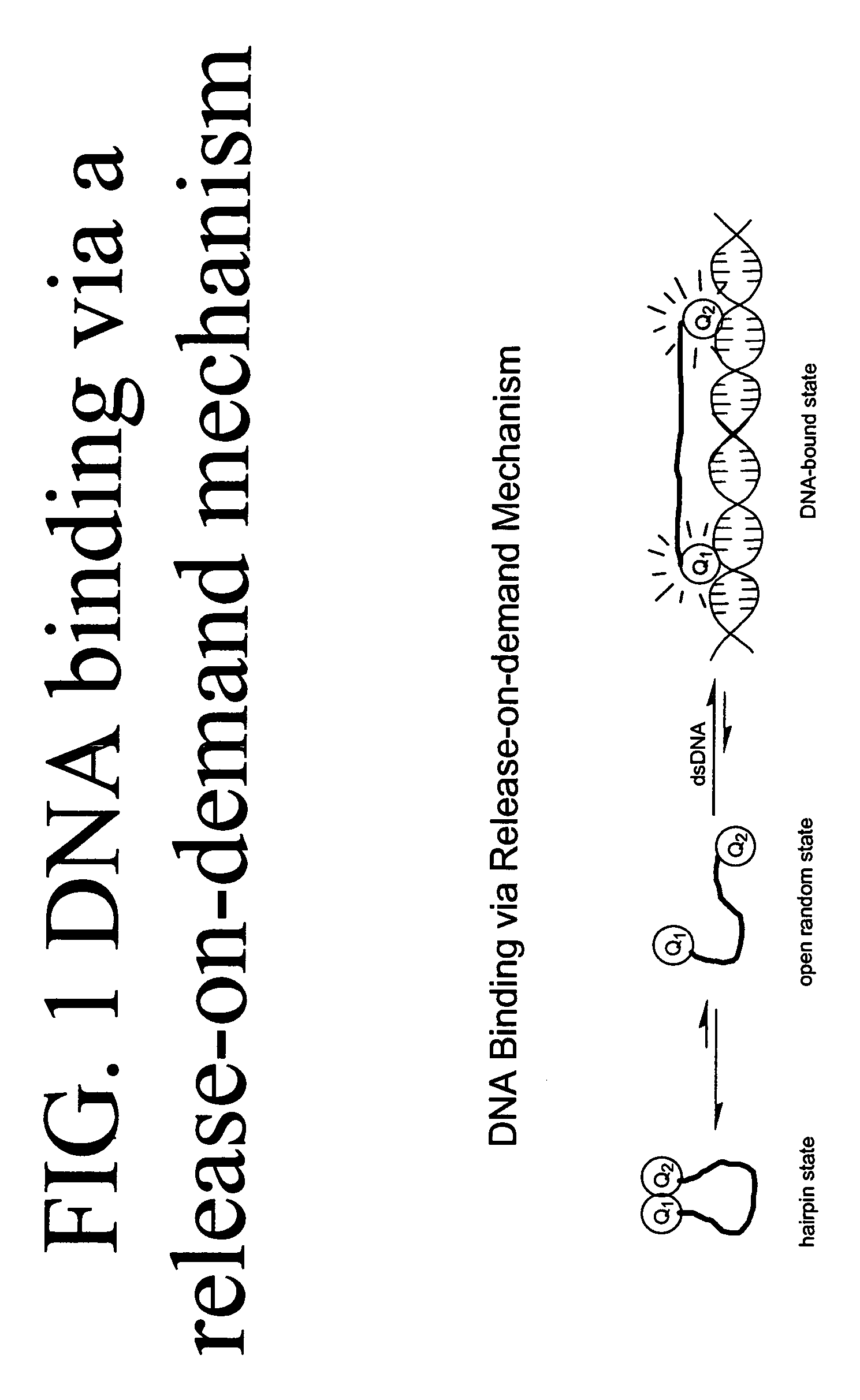
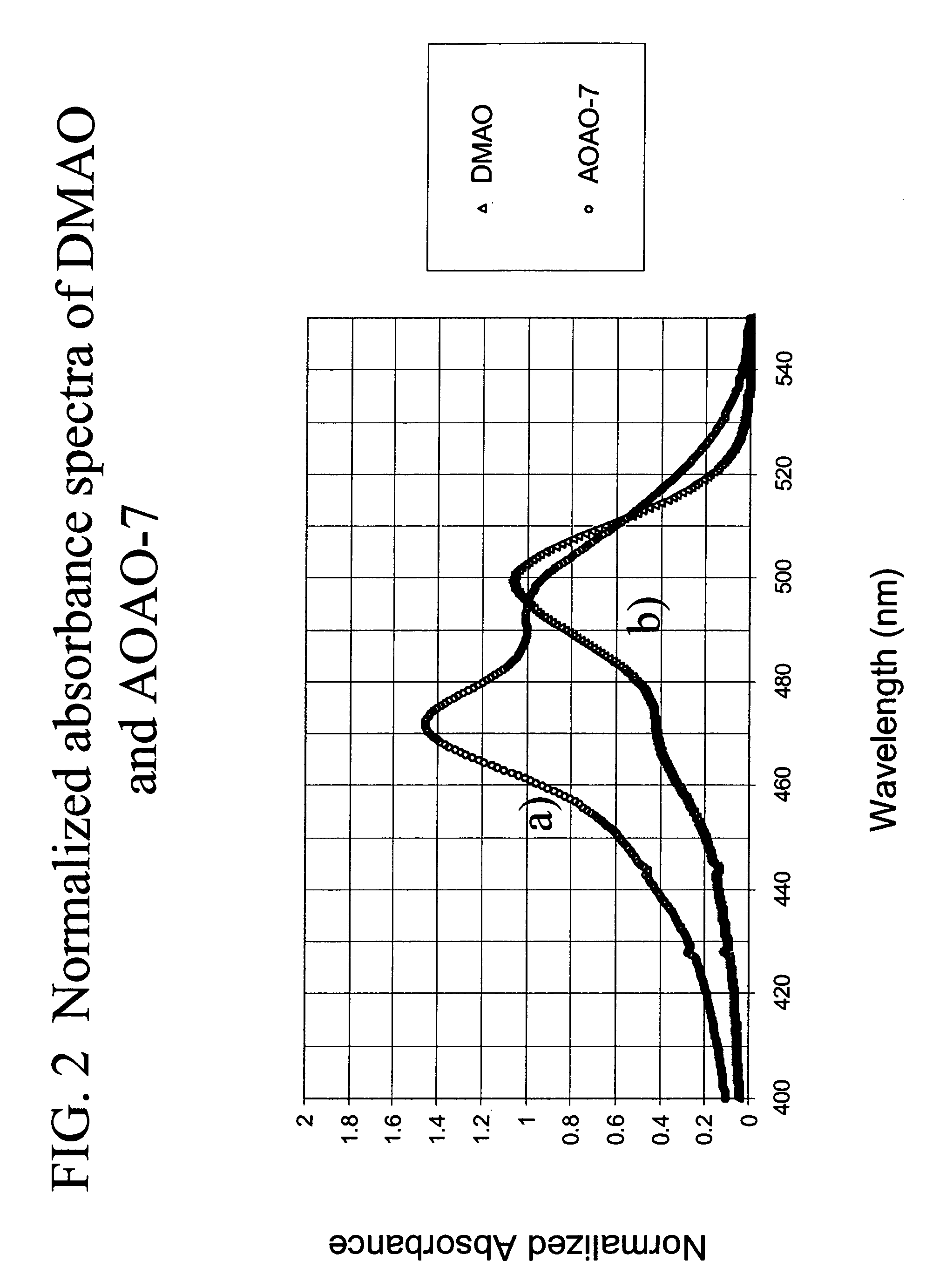
Methods of using dyes in association with nucleic acid staining or detection and associated technology
ActiveUS7601498B2Low toxicityIncrease signal strengthMethine/polymethine dyesSugar derivativesNucleic acid detectionStaining
Methods of using dyes and associated technology are provided. A dye, such as a monomeric dye or a dimeric dye, may be used in a nucleic acid gel staining application and / or a nucleic acid detection application. Such a dye and a salt that comprises an anion that is associated with a strong acid and a cation that is associated with a strong base may be used in such an application. A dimeric dye, such as a dimeric dye capable of forming a hairpin-like structure, may be used to stain and / or detect nucleic acids via a release-on-demand mechanism. A dimeric dye having low background fluorescence in the absence of nucleic acids and high fluorescence in the presence of nucleic acids, upon binding therewith, may be used to stain and / or detect nucleic acids.
Owner:BIOTIUM INC
Method of producing alkylbenzene and catalyst used therefor
InactiveUS20120000819A1Increase added valueInhibit productionAluminium compoundsMolecular sieve catalystsAlkaneAcid strength
A method that efficiently produces an alkylbenzene with a high added value from a 1.5-cyclic aromatic hydrocarbon while suppressing excessive hydrocracking and nuclear hydrogenation, and preventing a decrease in catalytic activity due to deposition of carbon during a hydrocracking reaction, and a catalyst used therefor, are disclosed. A method of producing an alkylbenzene includes causing a hydrocarbon oil feedstock containing an alkylbenzene content of less than 20 vol %, a bicyclic aromatic hydrocarbon content of less than 30 vol %, and a 1.5-cyclic aromatic hydrocarbon content of 25 vol % or more to come in contact with a hydrocracking catalyst that includes a solid acid having a maximum acid strength of Brönsted acid of 110 kJ / mol or more and less than 140 kJ / mol.
Owner:GASOLINEEUM ENERGY CENT FOUND +1
High-temperature smoke selective catalytic reduction (SCR) denitration catalyst, preparation method and application thereof
ActiveCN101961656ALarge specific surface areaAvoid lostPhysical/chemical process catalystsDispersed particle separationAcid strengthCordierites
The invention discloses a high-temperature smoke selective catalytic reduction (SCR) denitration catalyst. The high-temperature smoke SCR denitration catalyst consists of a carrier and an active ingredient loaded on the carrier and is characterized in that: the carrier is a cordierite honey ceramic loaded with a TiO2-SiO2 coating; and the loading capacity of the TiO2-SiO2 coating is 5 to 30 weight percent. The high-temperature smoke SCR denitration catalyst is prepared by loading titanyl sulfate and silica sol serving as raw materials on a cordierite honey ceramic base material by an in-situ precipitation method so as to form the uniform and firm TiO2-SiO2 coating on the surface of the base material, wherein the obtained TiO2-SiO2 coating has high specific surface area and proper acid strength and can effectively promote catalysis.
Owner:ZHEJIANG UNIV OF TECH
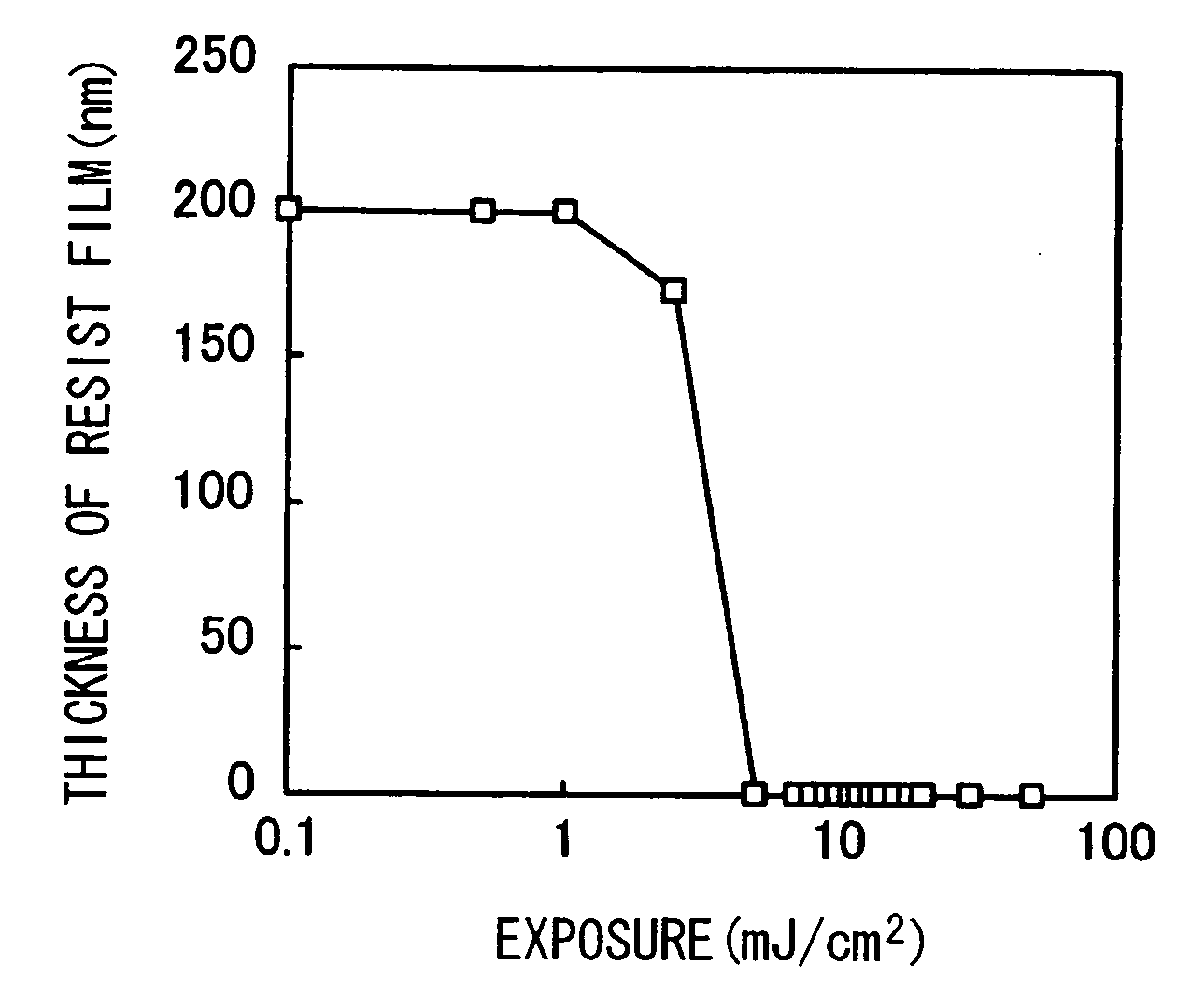
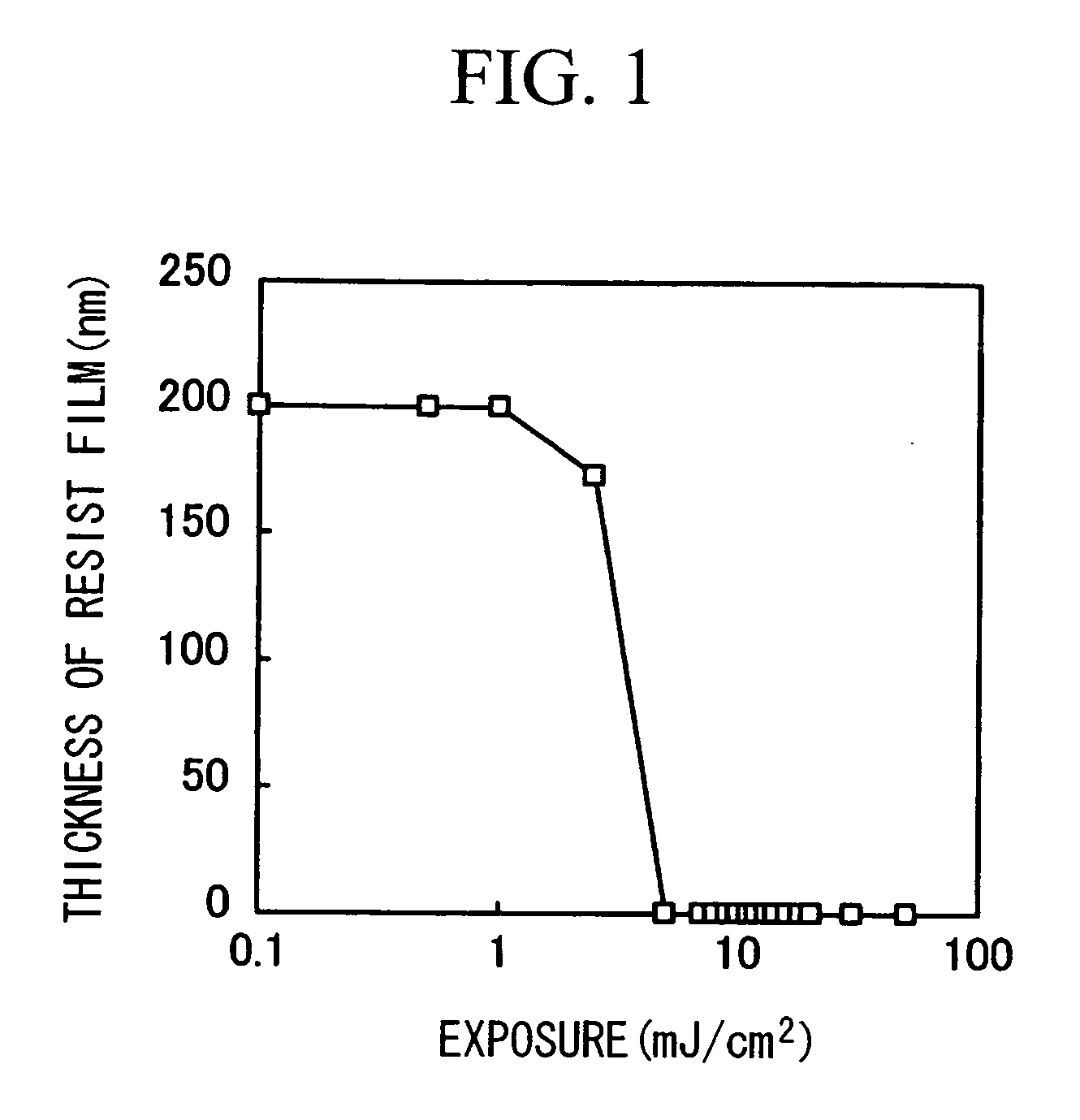
Thermal acid generator, resist undercoat material and patterning process
ActiveUS7514202B2Increase etch rateEtch rate reductionOrganic chemistryPhotosensitive materialsArylResist
A thermal acid generator of generating an acid on heating above 100° C. has formula: CF3CH(OCOR)CF2SO3−(R1)4N+ wherein R is alkyl or aryl, R1 is hydrogen, alkyl, alkenyl, oxoalkyl, aryl, aralkyl or aryloxoalkyl, or R1 may bond together to form a ring with N. The sulfonic acid generated possesses an ester site within molecule so that less bulky acyl groups to bulky groups may be incorporated therein. The thermal acid generator provides a sufficient acid strength, is less volatile due to a high molecular weight, and ensures film formation. Upon disposal of used resist liquid, it may be converted into low accumulative compounds.
Owner:SHIN ETSU CHEM IND CO LTD
Method for recovering ruthenium catalyst carried by active carbon
InactiveCN1872418AReduce manufacturing costReduce consumptionRuthenium/rhodium/palladium/osmium/iridium/platinum compoundsCatalyst regeneration/reactivationRecovery methodActivated carbon
A recovering process for the activated carbon carried Ru catalyst not containing the compound of alkali metal or alkali-earth metal includes such steps as calcining at 600-1000 deg.C for 2-20 hr to obtain gray mixture, mixing it with KOH and KNO3, holding the temp at 300-950 deg.C for 105 hr, cooling, dissolving in hot water at 50-90 deg.C to obtain K2RuO4 solution, adding sodium hypochlorite and concentrated sulfuric acid, distilling to generate RuO4 gas, absorbing it by strong acid solution, and distilling to obtain Ru salt.
Owner:ZHEJIANG UNIV OF TECH +1
Catalyst used for hydrogenation ring opening reaction of polycyclic aromatic hydrocarbon, and preparation method and application thereof
InactiveCN104117386AAchieve conversion rateLower cold filter pointMolecular sieve catalystsHydrocarbon oils treatmentIsomerizationAcid strength
The invention relates to a catalyst used for hydrogenation ring opening of polycyclic aromatic hydrocarbon and advanced hydrogenation dearomatization and isomeric pour point depression of diesel oil, and a preparation method thereof. The catalyst is formed by using modified H-Beta molecular sieve with a high silica-alumina ratio and an inorganic oxide as a carrier, and precious metal Pt, Pd or Ir as an active component. The catalyst has a high metal dispersion ability, and has suitable acid kind, acid strength and pore structure. The catalyst can inhibit advanced cracking, and promotes the selective ring opening of aromatic hydrocarbon and product isomerization. The catalyst is suitable for advanced hydrogenation saturation of diesel oil with low sulfur and nitrogen content, selective ring opening and isomerization, can reduce the aromatic hydrocarbon content in the diesel oil, can improve the cetane number of oil products, and can reduce the condensation point of the diesel oil.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Thermal acid generator, resist undercoat material and patterning process
ActiveUS20070264596A1Increase etch rateEtch rate reductionOrganic chemistryPhotosensitive materialsResistAryl
A thermal acid generator of generating an acid on heating above 100° C. has formula: CF3CH(OCOR)CF2SO3−(R1)4N+ wherein R is alkyl or aryl, R1 is hydrogen, alkyl, alkenyl, oxoalkyl, aryl, aralkyl or aryloxoalkyl, or R1 may bond together to form a ring with N. The sulfonic acid generated possesses an ester site within molecule so that less bulky acyl groups to bulky groups may be incorporated therein. The thermal acid generator provides a sufficient acid strength, is less volatile due to a high molecular weight, and ensures film formation. Upon disposal of used resist liquid, it may be converted into low accumulative compounds.
Owner:SHIN ETSU CHEM IND CO LTD
Low-temperature flue gas SCR (Selective Catalytic Reduction) denitrating catalyst and preparation method and application thereof
ActiveCN102019187ALarge specific surface areaHighly active phaseDispersed particle separationCatalyst activation/preparationSlurryAcid strength
The invention discloses a low-temperature flue gas SCR (Selective Catalytic Reduction) denitrating catalyst, comprising a carrier and an active component loaded on the carrier, wherein the carrier is cordierite honeycomb ceramic loaded with a TiO2-SiO2 coating; the ratio of the amount of substances of Ti to Si in the TiO2-SiO2 coating is 1:0.1-2.0; the active component is an oxide composed of Mn,Fe, Ce, Zr, and W; the loading amount of the active component is 2-20 wt%; the ratio of the amount of substances of Mn to Fe to Ce to Zr to W in the active component is 1.0:0.1-2.0:0.1-2.0:0.02-1.0:0.01-1.0. In the invention, a uniform and firm TiO2-SiO2 coating is formed by using in situ precipitation method; the obtained coating has high specific surface area and appropriate acid strength, and can effectively play promoting catalytic action role; and the active component can be prepared by using in situ coprecipitation method. The preparation process avoids the problems that the seriflux isin serious loss and the coating is not uniform during the suspension dip-coating, and maintains the highly activity phase of the composite oxide.
Owner:ZHEJIANG UNIV OF TECH
Purification method of ultra-pure silicon dioxide sol
InactiveCN101475180AHigh purityGood removal effectSilicon compoundsPolishing compositions with abrasivesAlkali ionsPurification methods
The invention discloses a method for purifying a silica sol with superhigh purity in the technical field of chemical mechanical polishing. The purification process comprises taht: strong acid type cation exchange resin, strong alkali type anion exchange resin and strong acid and strong alkali mixed-bed resin are filled to an ion exchange column and are subjected to regeneration treatment; the temperature of the silica sol is controlled between 0 and 60 DEG C and inversely flows through a strong acid type cation exchange bed; the obtained acid silica sol inversely flows through a strong alkali type anion exchange bed and is added with a chelator so that pH of the silica sol is between 1.0 and 3.0; and the silica sol added with the chelator inversely flows through a strong acid and strong alkali ion exchange resin mixed bed to obtain the purified silica sol. The method has good purification effect, can obtain the silica sol of which the superhigh purity meets the requirement of the chemical mechanical polishing, has short purification time and high efficiency, and can be used for continuous industrialized production.
Owner:TSINGHUA UNIV +1
Method for preparing high-purity phosphotungsticacid
ActiveCN1978327ALow in phosphateHigh purityPhysical/chemical process catalystsTungsten compoundsPhosphoric acidSodium tungstate dihydrate
This invention introduces a method for preparing pure PTA with sodium wolframate as the raw material including : 1, contacting sodium wolframate solution with inorganic acid solution till depositing active tungstenic acid, 2, contacting the deposition with phosphoric acid till dissolving the active tungstenic completely to get PTA solution, 3, adding an inorganic acid precipitator to the solution to deposit, filter and crystallize the deposition to get pure PTA, in which, the mol concentration of the precipitator in the system is 0.1-6mol / 1.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing non-crystal molybdemum sulfide nano powder
InactiveCN101024517ARapid and uniform precipitation reactionShort reaction timeNanostructure manufactureMolybdenum sulfidesSolubilityStrong acids
The invention relates to a manufacture method for amorphous form molybdenum sulphide nanometer powder. The feature is that: it uses solubility molybdenate, thiacetamide, strong acid and dispersing agent as raw material, dissolving solubility molybdenate and thiacetamide into deionized water, adding dispersing agent, adding strong acid in a short time, reacting at constant temperature, absorbing tail gas hydrogen disulfide by NaOH sulotion, filtering, washing, drying the depositing to gain MoSx(=2-4) nanometer powder, using it as precursor, burning and gaining amorphous form molybdenum sulphide nanometer powder that has widely application prospect in lubrication, catalyst and photo-electric-magnetism field.
Owner:HEFEI UNIV OF TECH
Cubic mesoporous molecular sieve catalyst with micropore canals, preparation method and use thereof
InactiveCN101683620AHigh catalytic activityGood choiceMolecular sieve catalystsHydrocarbonsMicrowaveSynthesis methods
The invention relates to a cubic mesoporous molecular sieve catalyst with micropore canals, a preparation method and use thereof. The catalyst comprises the raw materials of an aluminium source, a silicon source, sodium hydroxide, a template, surfactant and de-ionized water, and is prepared through the following steps: firstly preparing a silicon-aluminium precursor; and then utilizing a self-assembly function between the precursor and the surfactant to obtain stable molecular sieve materials. The preparation method comprises two methods of hydro-thermal synthesis and microwave synthesis. Dueto the adoption of the microwave synthesis method, the cubic mesoporous molecular sieve catalyst in the invention has the advantages of shortening the crystallization time, obtaining a synthesized molecular sieve with a cubic mesoporous structure, having micropore canals, having acid strength similar to that of a ZSM-5 molecular sieve, and having relatively higher catalytic activity when used forthe catalytic cracking reaction of 1,3,5-tri-isopropyl benzene.
Owner:BEIJING INSTITUTE OF PETROCHEMICAL TECHNOLOGY
Method for synthesizing silicoaluminophosphate molecular sieve by utilizing crystallization mother liquor
InactiveCN101935050AAdjust grainAdjust acid strengthMolecular-sieve and base-exchange phosphatesMolecular-sieve silicoaluminophosphatesMolecular sievePhysical chemistry
The invention discloses a method for synthesizing a silicoaluminophosphate molecular sieve by utilizing crystallization mother liquor, which comprises the following steps: 1) mixing an aluminum source with deionized water for forming suspension A, or mixing the aluminum source with the crystallization mother liquor for forming the suspension A; 2) mixing a phosphorus source with the deionized water for forming solution B, or mixing the phosphorus source with the crystallization mother liquor for forming the solution B; 3) mixing the suspension A with the solution B, and then adding a template agent, a silicon source, crystal seeds and the crystallization mother liquor for forming initial gel mixture; 4) placing the initial gel mixture in a crystallization kettle for hydrothermal crystallization, and then filtering for obtaining a filter cake and filtrate; 5) washing the filter cake, then drying and then calcining for obtaining the silicoaluminophosphate molecular sieve; and 6) recovering the filtrate, sealing and preserving. The method can reduce the using quantity of the template agent, realize zero emission of the crystallization mother liquor, and simultaneously regulate the grain size and the acid strength of the silicoaluminophosphate molecular sieve by fully utilizing the crystallization mother liquor.
Owner:THE NORTHWEST RES INST OF CHEM IND +1
A kind of boron-containing zsm-5 zeolite catalyst for producing olefin from methanol and preparation method thereof
InactiveCN102259013AHigh activitySolution to short lifeMolecular sieve catalystsHydrocarbon from oxygen organic compoundsMolecular sievePtru catalyst
The invention belongs to the technical field of chemical catalysts, in particular to a boron-containing zeolite catalyst for preparing olefins from methanol, and a preparation method and application thereof. In the invention, the silicon source, the aluminum source, the boron source and the template are mixed with deionized water and subjected to hydrothermal treatment to synthesize a ZSM-5 zeolite catalyst containing both boron and aluminum and silicon in the framework in one step. The synthesized zeolite has a high crystallinity and a typical MFI structure, and the medium acid strength and the ratio of strong acid sites on the surface of the molecular sieve can be effectively controlled. The zeolite catalyst exhibits excellent catalytic activity and stability in the catalytic reaction of methanol to olefins.
Owner:FUDAN UNIV +1
Stable wet strength resin
ActiveUS7291695B2Improve stabilityImprove performanceNatural cellulose pulp/paperSpecial paperStrong acidsProton
A method of stabilizing an aqueous solution of polyaminoamide-epichlorohydrin resin comprising adjusting the pH of the solution to less than about 3 with strong acid and then adding to the solution a weak acid in a mole ratio of total protons available from the weak acid to total protons available from the strong acid of about 0.05 to about 2.
Owner:ECOLAB USA INC
Preparation method of multi-component organic cross-linked acid liquid
The invention relates to a preparation method of multi-component organic cross-linked acid liquid, which comprises base liquid and a cross-linking agent, wherein the weight ratio of the base liquid and the cross-linking agent is 100:0.6-0.8; the base liquid comprises the following components in percentage by weight: 3.6-9 percent of formic acid, 4.8-12 percent of acetic acid, 5.5-13.75 percent ofhydrochloric acid, 0.6-0.8 percent of thickening agent, 4.0-5.0 percent of high-temperature corrosion inhibitors, 0.5-1.0 percent of long-term clay stabilizer, 1.0-1.5 percent of ferric ion stabilizers, 0.5-1.0 percent of broken emulsion discharge aiding agent and the balance is water; and the sum of the mass percentages of all the components is 100%. The method has the beneficial effects that the acid liquid system can be cross-linked under the conditions of high temperature and strong acid and is good in temperature resistance and shear resistance, less in filtration and complete in gelout;after the acid liquid is cross-linked, an acid rock is remarkably lower than ordinary gelled acid in reaction speed and is good in speed retarding performance; and the multi-component organic cross-linked acid liquid can be used for realizing the modification of depth acid pressure in a high-temperature deep well of a carbonate rock.
Owner:BC P INC CHINA NAT PETROLEUM CORP +2
High-temperature-resistant crosslinking acid liquor system as well as preparation method thereof
ActiveCN104403658AFacilitate cross-linkingImprove temperature resistanceFluid removalDrilling compositionAcid fracturingCross-link
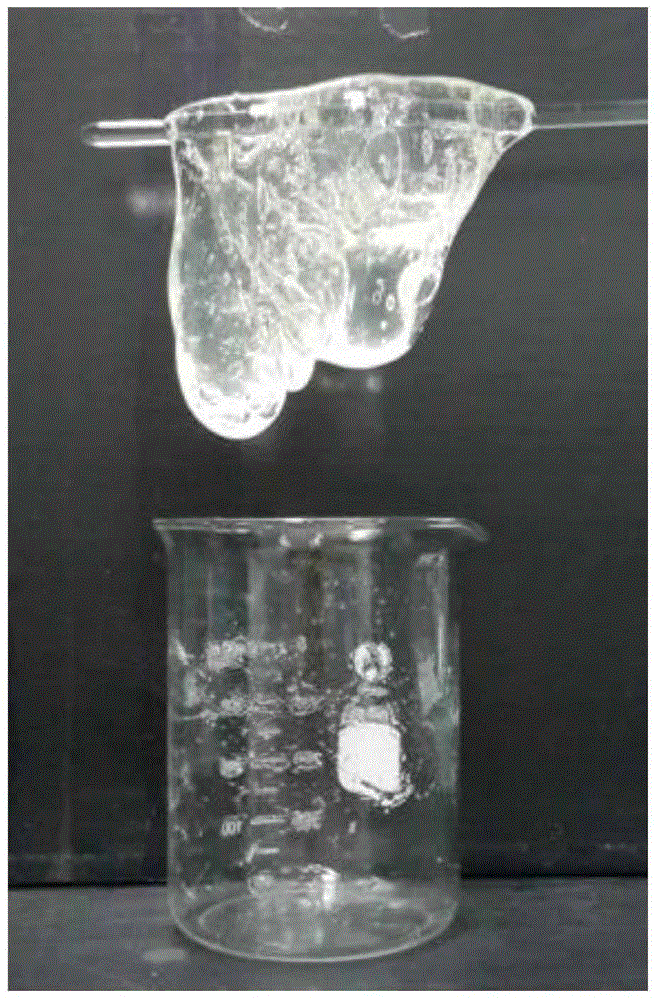
The invention relates to a high-temperature-resistant crosslinking acid liquor system as well as a preparation method thereof. The system is prepared from the following components in percentage by mass: 20% of hydrochloric acid (based on pure hydrogen chloride), 1% of a polymer thickener, 0.3% of an organic metal cross-linking agent chelated titanium cross-linking agent and the balance of water; the preparation method comprises the following steps: adding quantitative thickener into a hydrochloric acid solution at normal temperature; quickly stirring till being fully dissolved; then, performing standing for 2-4 hours to obtain a uniform viscous liquid; finally adding the organic metal cross-linking agent and uniformly stirring. The system provided by the invention is less in use level of chelated titanium cross-linking agent, low in cost and good in stability, and no precipitates occur after being placed for a long time. The obtained high-temperature cross-linking acid liquor is crosslinked under a strong acid environment to form a jelly which can be picked up, and moreover, the obtained high-temperature-resistant crosslinking acid liquor system can resist a high temperature of 190 DEG C and can be used for acid fracturing production increase of a carbonatite oil and gas reservoir at 160-190 DEG C.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Boron and nitrogen co-doping chitosan-base activated carbon and method for preparing same
ActiveCN102951637AHigh nitrogen contentHigh specific capacitanceCarbon compoundsProtonationStrong acids
The invention provides a boron and nitrogen co-doping activated carbon which is prepared through utilizing biomass. The boron and nitrogen co-doping chitosan-base activated carbon takes chitosan and boric acid as raw materials, wherein boron in the activated carbon accounts for 1-3wt%, nitrogen in the activated carbon accounts for 4.1-9.2wt%, the specific surface area is 500-1000m<2> / g, and the pore size distribution is 0.46-2nm. The boron and nitrogen co-doping chitosan-base activated carbon takes the chitosan and the boric acid as raw materials and is prepared through co-pyrolysis. According to a method, the combining quantity of the boron in a carbon source and the dispersion degree of the boron in the carbon source are increased under the mutual action of the protonated chitosan and the boric acid, and the boron and nitrogen co-doping chitosan-base activated carbon is obtained through the co-pyrolysis. An environmental-friendly technology for preparing the activated carbon without consuming strong acid and strong alkali is realized, so that a costly and non-environmental-friendly process which consumes a great deal of activating agent and water during the traditional process for preparing the activated carbon is avoided. Moreover, the boron and nitrogen co-doping chitosan-base activated carbon has the advantages of short time consumption, simplicity in preparation technology, simplicity and easiness in equipment and the like.
Owner:DALIAN UNIV OF TECH
Non-porous single dispersed polymer weak cation exchange resin, its preparation method and use
A weak cationic exchange resin used for fast separating and purifying the genetic engineering products and protein is prepared through preparing the non-porous single-dispersed hydrophilic microspheres of cross-linked epoxy propyl polymethyl acrylate, and preparing said weak cationic exchange resin. Its advantages are easy regeneration, high resistance to strong acid and strong alkali, high separation speed and high recovery rate of protein.
Owner:NINGXIA UNIVERSITY
Anti-reflective coating composition with improved spin bowl compatibility
InactiveUS6962769B2Extended shelf lifeRadiation applicationsLayered productsAnti-reflective coatingAdditive ingredient
Anti-reflective compositions and methods of using those compositions to form circuits are provided. The compositions comprise a polymer dissolved or dispersed in a solvent system. In one embodiment, the compositions comprise less than about 0.3% by weight of a strong acid. In another embodiment, the weight ratio of strong acid to weak acid in the composition is from about 0:100 to about 25:75. Examples of preferred weak acid compounds include phenolic compounds (e.g., Bisphenol S, Bisphenol A, α-cyano-4-hydroxycinnamic acid), carboxylic acids (e.g., acetic acid), phosphoric acid, and cyano compounds. The polymer and other ingredients are preferably physically mixed in a solvent system. The resulting compositions are spin bowl compatible (i.e., they do not crosslink prior to the bake stages of the microlithographic processes or during storage at room temperature).
Owner:BREWER SCI
Process for scrubbing ammonia from acid gases comprising ammonia and hydrogen sulfide
Owner:EI DU PONT DE NEMOURS & CO
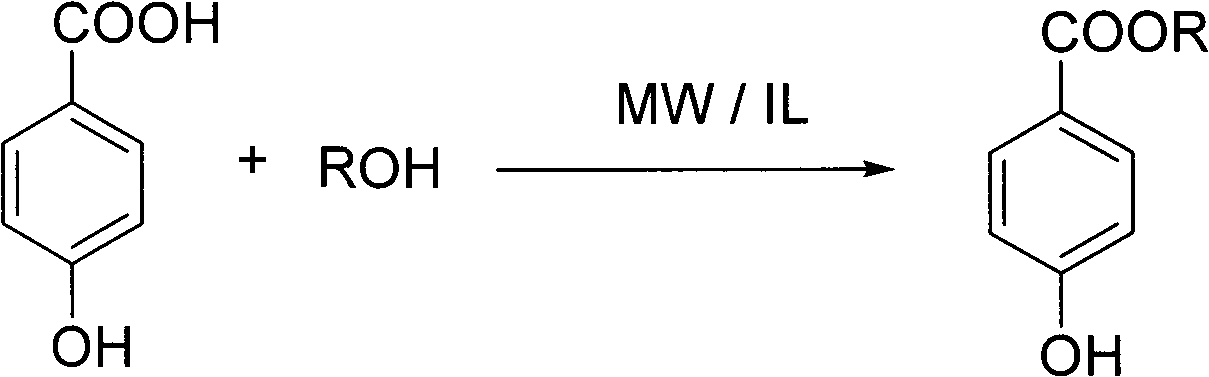
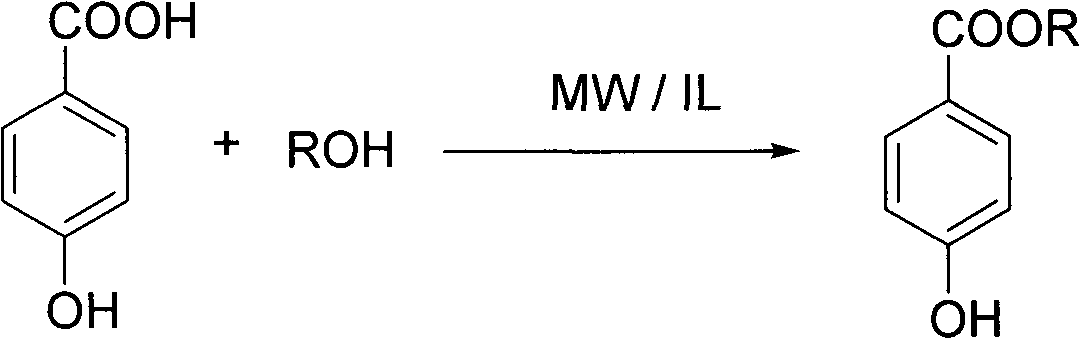
Preparation method of nipagin ester compound under promotion of sulfonic acidic ionic liquid
InactiveCN101982453AMild reaction conditionsAvoid corrosionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSodium bicarbonateStrong acids
The invention provides a preparation method of a nipagin ester compound under promotion of sulfonic acidic ionic liquid. The method comprises the following steps: taking the sulfonic acidic ionic liquid as a reaction solvent and a promoter, and carrying out esterification on p-hydroxybenzoic acid and corresponding alcohol at the temperature of 80-120 DEG C for 0.5-3h under a microwave radiation condition to obtain a coarse product of nipagin ester; adding water to the coarse product, and repeatedly extracting the product and impurities such as unreacted raw materials and the like with diethylether until a diethyl ether phase is non-fluorescent; merging the diethyl ether phase, sequentially washing with saturated sodium bicarbonate aqueous solution and saturated salt solution and drying with anhydrous sodium sulfate; removing the solvent through vacuum evaporation to obtain the nipagin ester product; and removing a large quantity of water from an aqueous phase by rotary evaporation, and drying with salicylic acid in vacuum to obtain the recovered ionic liquid which can be recycled. The preparation method has the advantages of mild reaction condition, simple batch feeding and aftertreatment, short reaction time, and high mole yield of the obtained finished product of the nipagin ester which is over 85%, and being capable of avoiding equipment corrosion and pollution caused by using strong acid.
Owner:SHANDONG UNIV +1
Imidazole onium salt anion-exchange membrane and preparation method thereof
InactiveCN102151502AImprove thermal stabilityGood chemical stabilitySemi-permeable membranesChemical structureHigh energy
The invention relates to an imidazole onium salt anion-exchange membrane and a preparation method thereof, belonging to an anion-exchange membrane. The invention provides an imidazole onium salt anion-exchange membrane with better thermal stability and chemical stability and a preparation method thereof. The imidazole onium salt anion-exchange membrane is prepared by adopting a polymeric membrane, and has a chemical structure of one class of high polymers with side chains containing imidazole onium salt. The imidazole onium salt anion-exchange membrane has a membrane ionic conductivity of being more than 0.01S / cm, has better thermal stability and chemical stability and can be used at high temperature and in a strong acid, strong alkali and strong oxidizing agent. The preparation method comprises the steps of: soaking a polymeric membrane in acetone, and drying to constant weight after cleaning; radiating a film by high-energy rays; placing the radiated film in a container, injecting monomers in the container to ensure that the film is completely immersed into the monomers, vacuumizing, charging inert gases to the container until no air exists in the container, placing a water bathfor reacting after sealing, removing unreacted monomers, and drying; and soaking the grafted film with a quaternization reagent, and washing with deionized water to obtain the imidazole onium salt anion-exchange membrane.
Owner:XIAMEN UNIV
Technology for producing fulvic acid fertilizer and high-strength corrugated paper by using whole cotton stalks as raw materials
ActiveCN108611912ASave resourcesIncrease productivityMachine wet endPulp beating/refining methodsHigh concentrationFiber
The invention provides a technology for producing a fulvic acid fertilizer and high-strength corrugated paper by using whole cotton stalks as raw materials. With the whole cotton stalks as the raw materials, by a weakly-acid imidized machine pulp pulping technology, through optimal combination of the cooking temperature, the cooking time and the pH value as well as high-concentration pulping and other pulping technological links, pulping red liquor rich in fulvic acid is prepared for producing the fertilizer, and high-performance pulp with a relatively light color is produced for producing thehigh-strength corrugated paper. By the technology, defects and shortcomings of a strong-alkali and strong-acid technology are avoided, differences in traits, components and structures of the whole cotton stalk raw materials are overcome, fulvic acid producing active components contained in cotton stalk cores, cotton stalk pulps, cotton boll shells and cotton stalk skins in the whole cotton stalksare effectively utilized, the advantage of combination of long and short whole cotton stalk fibers is effectively played for producing the high-strength corrugated paper, comprehensive utilization ofwhole cotton stalk resources is achieved, and a technological path for industrial and commercial utilization of the whole cotton stalk raw materials is opened up.
Owner:白博
Method for preparing condensing multi-kernel aromatic resin carbon based solid acid catalyst
InactiveCN101147880AImprove thermal stabilityHigh catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsStrong acidsSolid acid
The present invention discloses a preparation method of new-type condensed polynuclear aromatic resin carbon base solid acid catalyst. Said preparation method includes the following steps: using polycyclic aromatic compound or its mixture as raw material, using aldehyde compound as cross-linking agent, using inorganic strong acid as catalyst, making condensation reaction to obtain B-stage condensed polynuclear aromatic resin; adding inorganic strong acid and aldehyde compound in the B-stage condensed polynuclear aromatic resin, making continuous reaction at high temperature to obtain C-stage condensed polyunuclear aromatic resin; then making the C-stage condensed polynuclear romatic resin undergo the processes of sulfonation, washing by using water, filtering, removing sulfonating agent and drying so as to obtain the invented target product.
Owner:EAST CHINA NORMAL UNIV
Support for gas-phase oxidation catalyst and process for its production, gas-phase oxidation catalyst, and process for producing acrylic acid
ActiveUS7456129B2Easy to handleHigh yieldOrganic compound preparationOther chemical processesGas phaseCatalytic oxidation
A support for a gas-phase oxidation catalyst, the support including a solid acid, of which acid strength (H0) meets an inequality: −5.6≦H0≦1.5; a gas-phase oxidation catalyst including the above support and a complex oxide containing molybdenum and vanadium as essential components, the complex oxide being supported on the support; a process for producing acrylic acid by gas-phase catalytic oxidation of acrolein with molecular oxygen, the process including carrying out the gas-phase catalytic oxidation in a presence of the above gas-phase oxidation catalyst; and a process for producing the above support, the process including controlling an acid strength (H0) of a solid acid so as to meet an inequality: −5.6≦H0≦1.5 by adjusting a calcination temperature in a preparation of the solid acid contained in the support.
Owner:NIPPON SHOKUBAI CO LTD
Polymer Compound, Photoresist Composition Including the Polymer Compound, and Resist Pattern Formation Method
ActiveUS20080166655A1Good resist pattern shapeExquisite patternOrganic chemistryOrganic compound preparationPolymer scienceHalogen
The present invention provides a polymer compound which can constitute a photoresist composition which is capable of having an excellent resolution, forming a fine pattern with a good rectangularity, obtaining favorable resist characteristics even when acid strength of a acid generated from an acid generator is weak, and having favorable sensitivity; a photoresist composition including the polymer compound; and a resist pattern formation method using the photoresist composition. The photoresist composition and the resist pattern formation method use the polymer compound including an alkali soluble group (i), wherein the alkali soluble group (i) is at least one substituent group selected from an alcoholic hydroxyl group, a carboxyl group, or a phenolic hydroxyl group, and the substituent group is protected by an acid dissociable, dissolution inhibiting group (ii) represented by a general formula (1):—CH2—OCH2nR1 (1)(wherein R1 represents a cycloaliphatic group which contains no more than 20 carbon atoms and may contain an oxygen atom, a nitrogen atom, a sulfur atom, or a halogen atom, and n represents 0 or an integer of 1 to 5.).
Owner:TOKYO OHKA KOGYO CO LTD
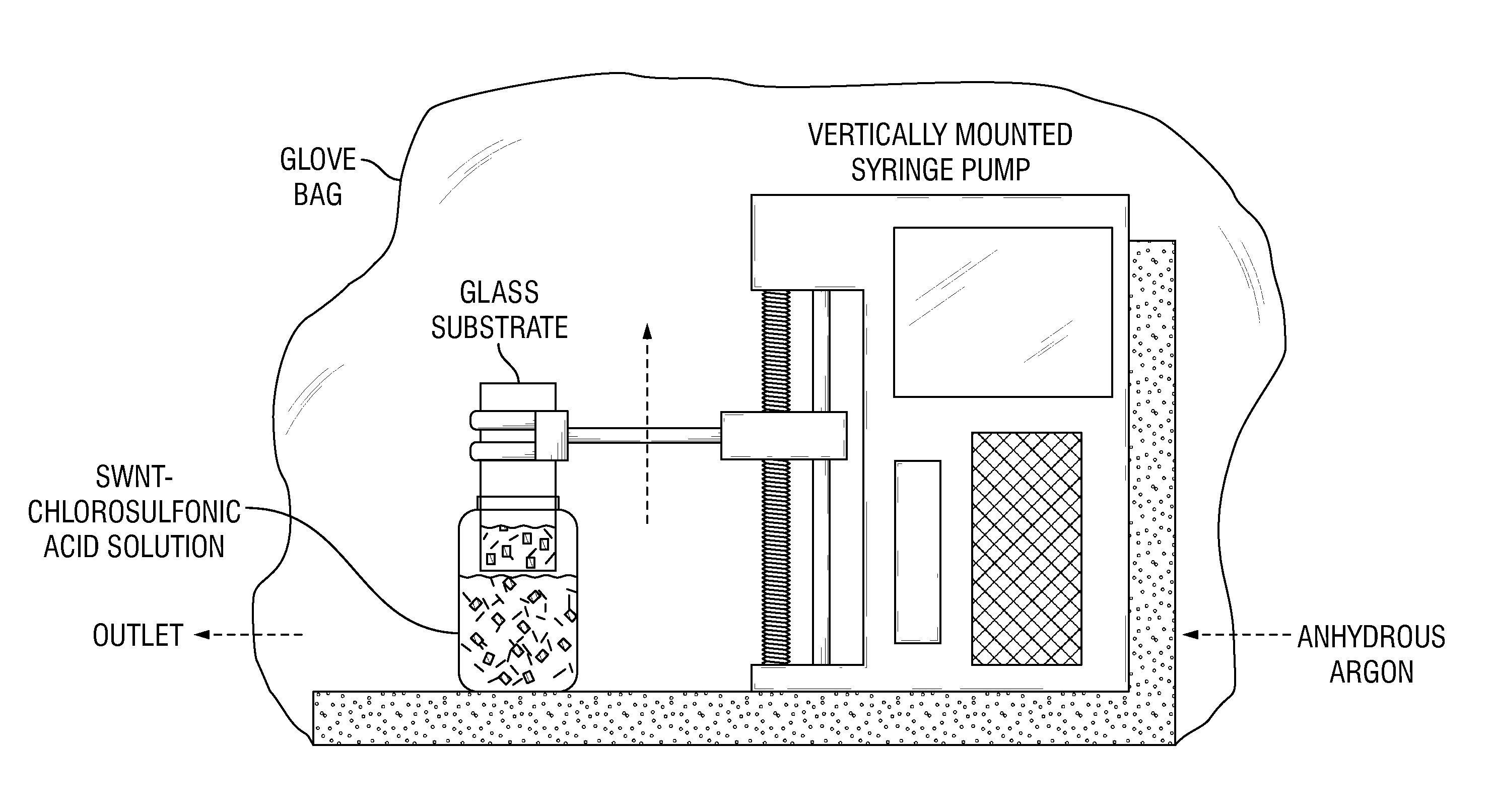
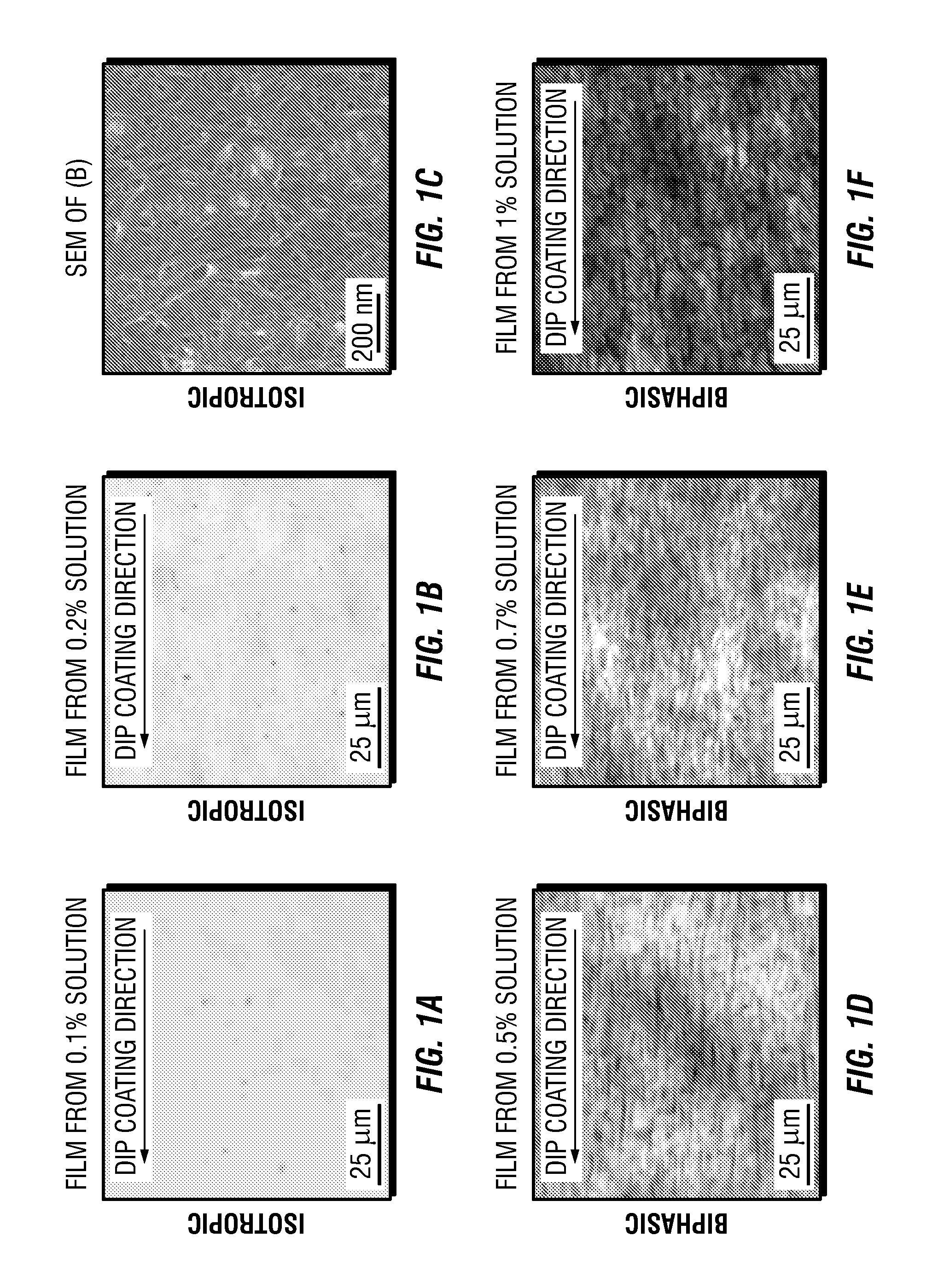
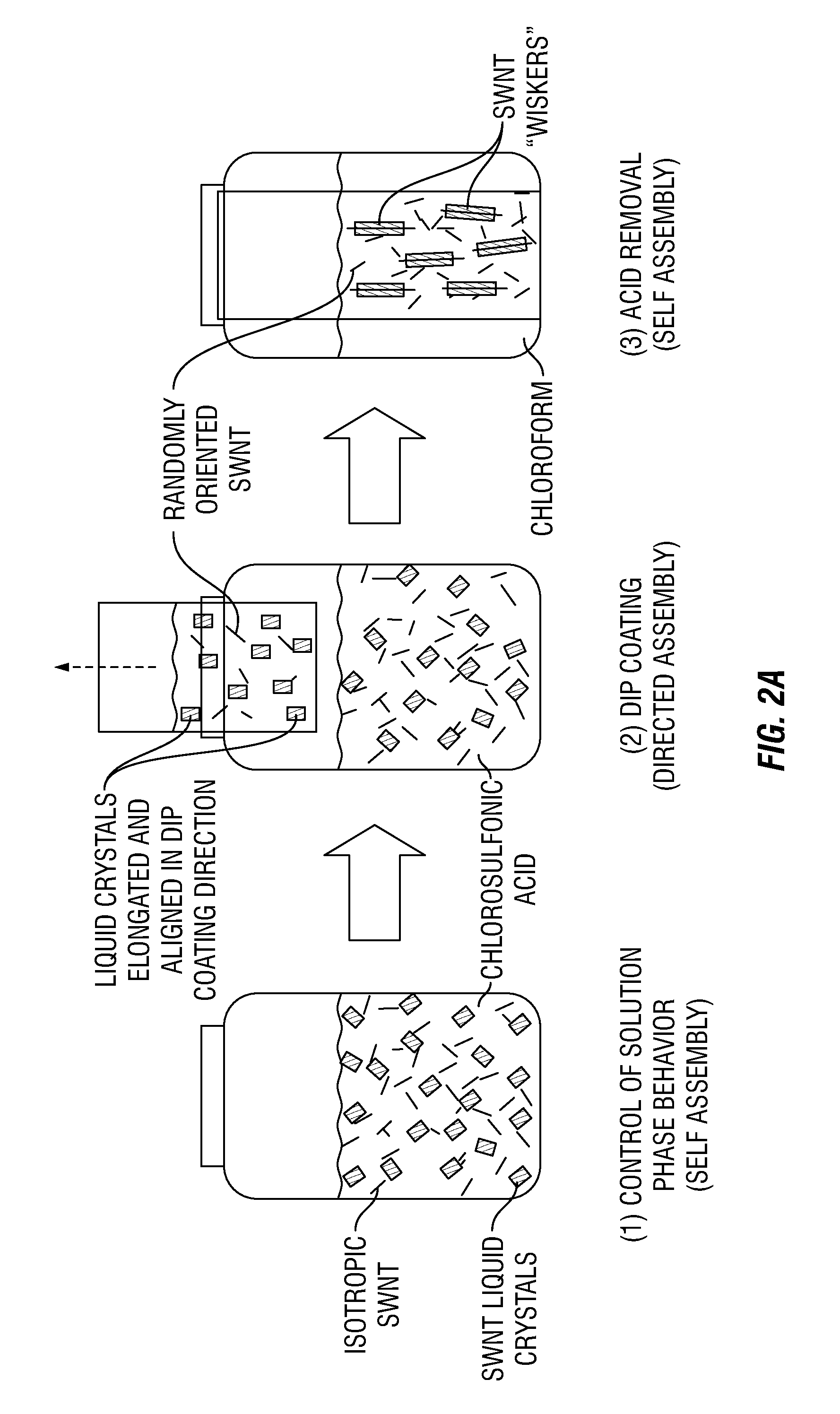
Carbon nanotube films processed from strong acid solutions and methods for production thereof
InactiveUS20150298164A1Material nanotechnologySynthetic resin layered productsStrong acidsCarbon nanotube
In some embodiments, the present disclosure provides methods for fabricating carbon nanotube films. Such methods generally comprise: (i) suspending carbon nanotubes in a superacid (e.g. chloro sulfonic acid) to form a dispersed carbon nanotube-superacid solution, wherein the carbon nanotubes have substantially exposed sidewalls in the carbon nanotube-superacid solution; (ii) applying the dispersed carbon nanotube-superacid solution onto a surface to form a carbon nanotube film; and (iii) removing the superacid. Desirably, such methods occur without the utilization of carbon nanotube wrapping molecules or sonication. Further embodiments of the present disclosure pertain to carbon nanotube films that are fabricated in accordance with the methods of the present disclosure. Such carbon nanotube films comprise a plurality of carbon nanotubes that are dispersed and individualized. Additional embodiments of the present disclosure pertain to macroscopic objects comprising the carbon nanotube films made in accordance with the methods of the present disclosure described supra.
Owner:RICE UNIV
N-butane isomerization catalyst and preparation method thereof
InactiveCN107051420AHigh acid strengthHigh activityCatalyst carriersHydrocarbon by isomerisationSulfate radicalsIsomerization
The invention provides an n-butane isomerization catalyst and a preparation method thereof. The preparation method comprises the following steps: preparing hydroxide by using a coprecipitation method, introducing lanthanum salt to increase the ratio of a tetragonal phase, performing hydro-thermal treatment, drying so as to obtain zirconium oxide which is sufficiently crystallized into the tetragonal phase, soaking to load a sulfate radical and molybdate, roasting so as to increase the acid strength of the catalyst, molding, further soaking into a VIII group metal, and performing synthesis, thereby obtaining the n-butane isomerization catalyst which adopts the tetragonal phase zirconium oxide as a carrier. The n-butane isomerization catalyst adopts the nano-grade tetragonal phase zirconium oxide as the carrier; on the basis of the dry basis of the tetragonal phase zirconium oxide carrier, the n-butane isomerization catalyst comprises the following active components in percentage by mass: 0.5-5.0% of lanthanum, 0.5-2.5% of sulfur, 0.5-2.5% of molybdenum and 0.01-5.0% of the VIII group metal. The n-butane isomerization catalyst is high in isomerization activity and selectivity, good in repeatability and catalysis stability, low in reaction temperature, and still relatively high in n-butane isomerization activity at high temperature.
Owner:CHINA UNIV OF PETROLEUM (BEIJING)
Preparation method of rare-earth modified MCM-48 loaded double-function catalyst
InactiveCN103551192AEvenly distributedReduce acid strengthHydrocarbon by isomerisationOrganic-compounds/hydrides/coordination-complexes catalystsMolecular sieveNitrate
The invention relates to a preparation method of a rare-earth modified MCM-48 loaded double-function catalyst. The method comprises the following steps of mixing and ultrasonically oscillating tetraethoxysilane ethyl orthosilicate and deionized water, adding the mixture into 4.5 mol / L sodium hydroxide solution, then adding 0.27 mol / L cerous nitrate, ultrasonically oscillating the solution, adding cetyl-trimethyl ammonium bromide, and ultrasonically oscillating the mixed solution to obtain mixture gel with the ratio of 1.0TEOS: 0.65CTAB: 0.5NaOH:62H2O:0.03Ce; after standing and crystallizing, low-pressure filtering, washing and drying the gel to obtain Ce-MCM-48 raw powder, and roasting the raw powder to obtain a catalyst carrier Ce-MCM-48 mesoporous molecular sieve; soaking the powder Ce-MCM-48 mesoporous molecular sieve by using a phosphotungstic acid solution, and drying and roasting the mesoporous molecular sieve to obtain a Ni-HPW / Ce-MCM-48 catalyst precursor. By adopting the preparation method, the difficulties of the prior art after an MCM-48 skeleton is doped into the rare earth, the specific surface area is reduced, the pore canals are blocked by the rare earth in a form of rare-earth oxide, the pore volume is small, the acid strength is low and the like can be solved.
Owner:NORTHEAST GASOLINEEUM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com