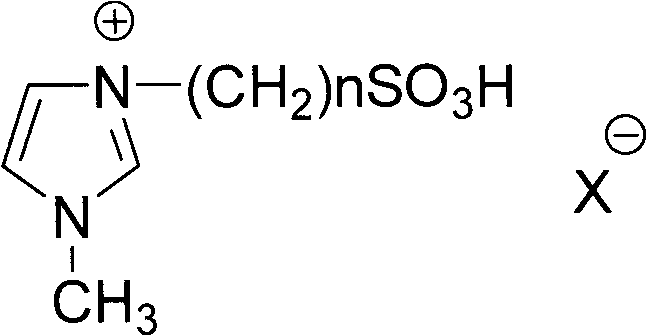Preparation method of nipagin ester compound under promotion of sulfonic acidic ionic liquid
A technology of parabens and ionic liquids, which is applied in the preparation of organic compounds, the preparation of carboxylic acid esters, chemical instruments and methods, etc. The effect of mild production and reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
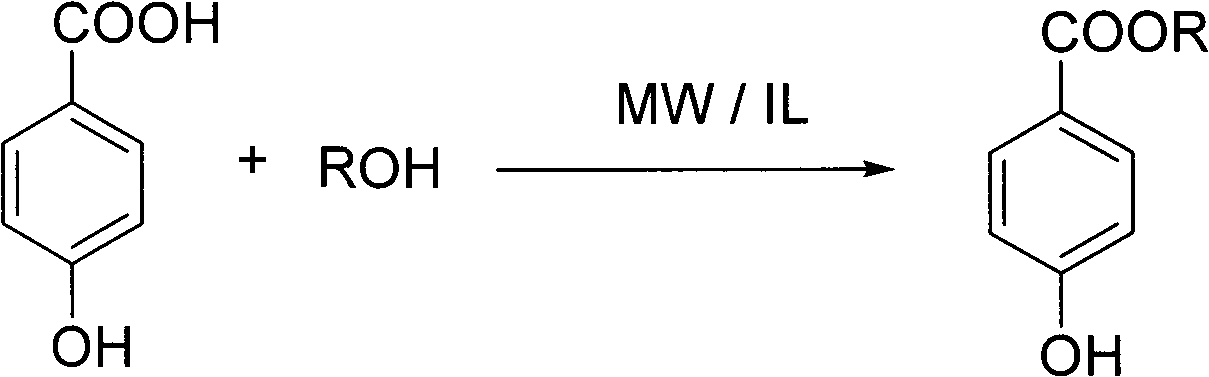
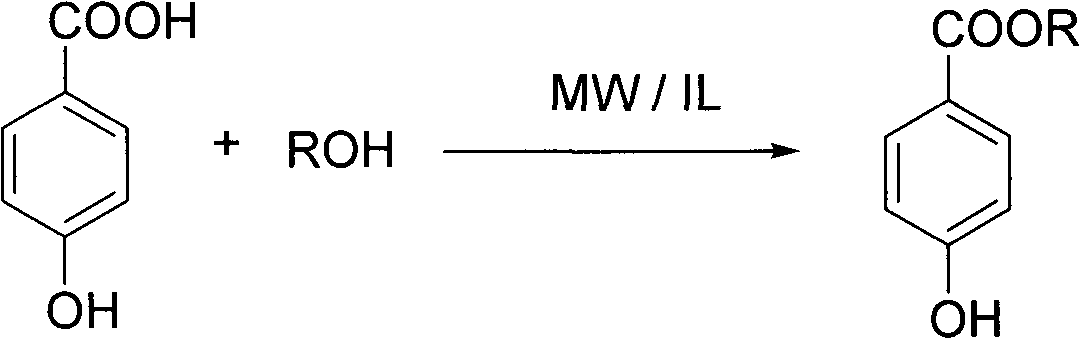
[0034] The preparation reaction of parabens:
[0035]
[0036] R=CH 3 , C 2 h 5 , n-C 3 h 7 , n-C 4 h 9 , i-C 4 h 9 , n-C 7 h 15 , n-C 12 h25 , n-C 16 h 33 , n-C 18 h 36 , etc.
[0037] Concrete preparation reaction steps are as follows:
[0038] Using sulfonic acid-type ionic liquid as reaction solvent and accelerator, under the condition of microwave radiation, make p-hydroxybenzoic acid and corresponding alcohol undergo esterification reaction at 80-120°C for 0.5-3 hours, then extract, wash and concentrate Obtain paraben product.
[0039] Wherein, the ionic liquids serving as reaction solvent and accelerator are respectively N-(4-sulfonic acid group) butyl imidazolium hydrogen sulfate (IL1), N-(3-sulfonic acid group) propyl imidazole hydrogen sulfate (IL2) , one of N-(4-sulfonic acid) butylimidazole p-toluenesulfonate (IL3) or N-(3-sulfonic acid) propylimidazole p-toluenesulfonate (IL4); p-hydroxybenzoic acid and The molar equivalent ratio of the alcoh...
Embodiment 1
[0042] Add 3.036g (0.022mol) of p-hydroxybenzoic acid and 2mL of ionic liquid N-(4-sulfonic acid) butylimidazolium bisulfate (IL1) into a 25mL round bottom bottle, stir at room temperature for 20 minutes, then add 1.8 mL (0.020 mol) of n-butanol, the microwave radiation power was set to 500w, and the reaction mixture was heated to 100°C for 0.5 hour reaction. Then the reaction system was cooled to room temperature (such as 25° C.), diluted with an appropriate amount of water, and extracted repeatedly with ether (10 mL×4 times) until there was no fluorescent substance. Vigorous stirring is necessary during extraction to ensure that no product remains in the ionic liquid. The ether phases were combined, washed with saturated sodium bicarbonate solution and saturated brine, and the organic phase was separated and dried over anhydrous sodium sulfate. Ethyl ether was removed by rotary evaporation under reduced pressure to obtain white solid n-butylparaben with a melting point of 6...
Embodiment 2
[0044] Add 3.036g (0.022mol) of p-hydroxybenzoic acid and 2mL of ionic liquid N-(4-sulfonic acid) butylimidazolium bisulfate (IL1) into a 25mL round bottom bottle, stir at room temperature for 20 minutes, then add 0.020 mol of isobutanol, the microwave radiation power was set to 600w, and the reaction mixture was heated to 110° C. for 2 hours. Then the reaction system was cooled to room temperature (such as 25° C.), diluted with an appropriate amount of water, and extracted repeatedly with ether (10 mL×4 times) until there was no fluorescent substance. Vigorous stirring is necessary during extraction to ensure that no product remains in the ionic liquid. The ether phases were combined, washed with saturated sodium bicarbonate solution and saturated brine, and the organic phase was separated and dried over anhydrous sodium sulfate. Ether was removed by rotary evaporation under reduced pressure to obtain isobutylparaben as a white solid with a melting point of 73-74°C (literatu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com