Process for scrubbing ammonia from acid gases comprising ammonia and hydrogen sulfide
A sour gas and hydrogen sulfide technology, applied in chemical instruments and methods, separation methods, gas fuels, etc., can solve expensive and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
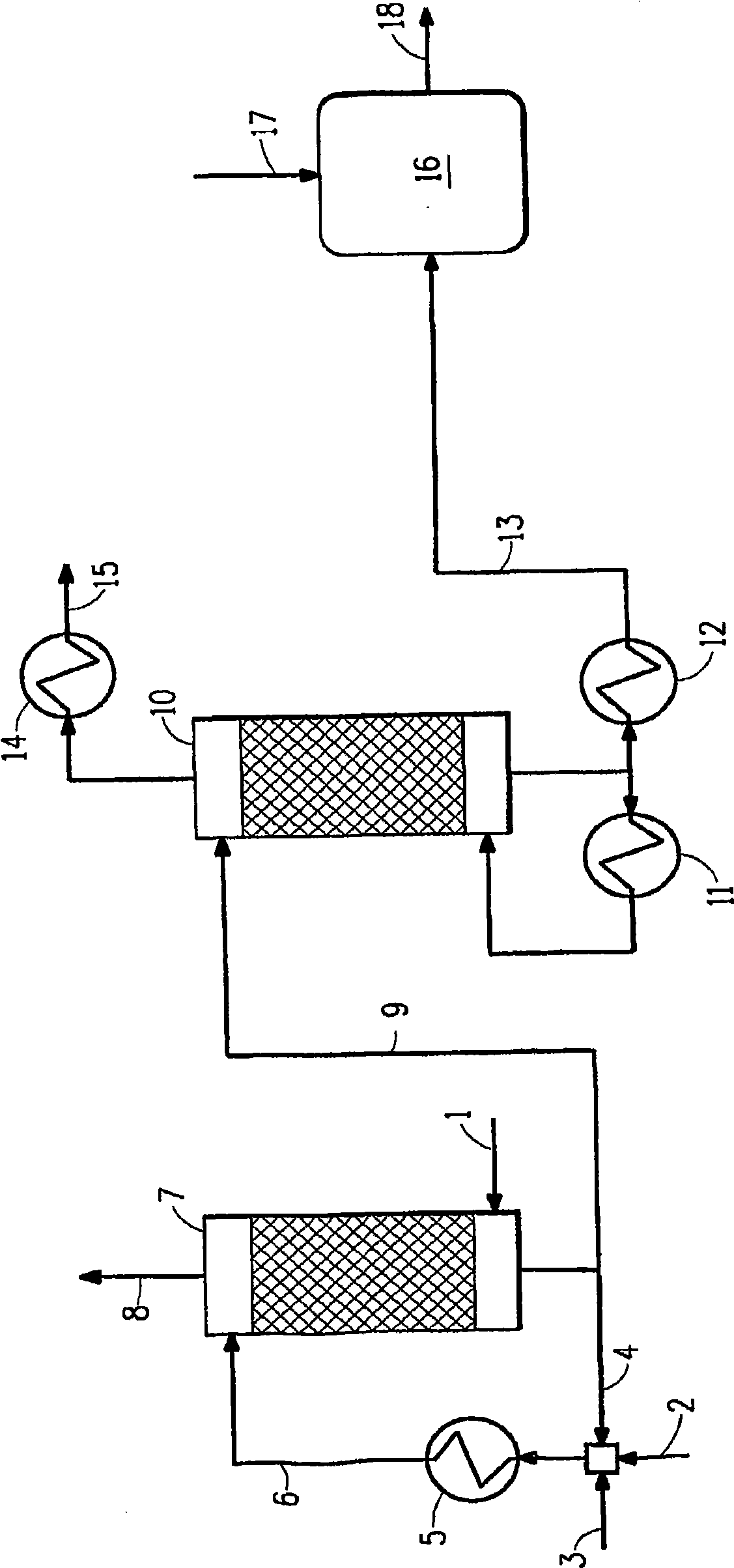
[0067] conduct figure 1 Computer modeling simulation of the method described in . A 143.3 lb mol / h (65 kgmol / h) sour water stripper containing 38.6 vol% ammonia (equivalent to 0.28 mass fraction) was used using recycled acid effluent (weak acid, including 5 wt% sulfuric acid) and fresh acid (98 wt% sulfuric acid) Scrubbing ammonia in the gas. Stream profiles and concentrations throughout the simulation for this example are shown in Table 1 below.
Embodiment 2
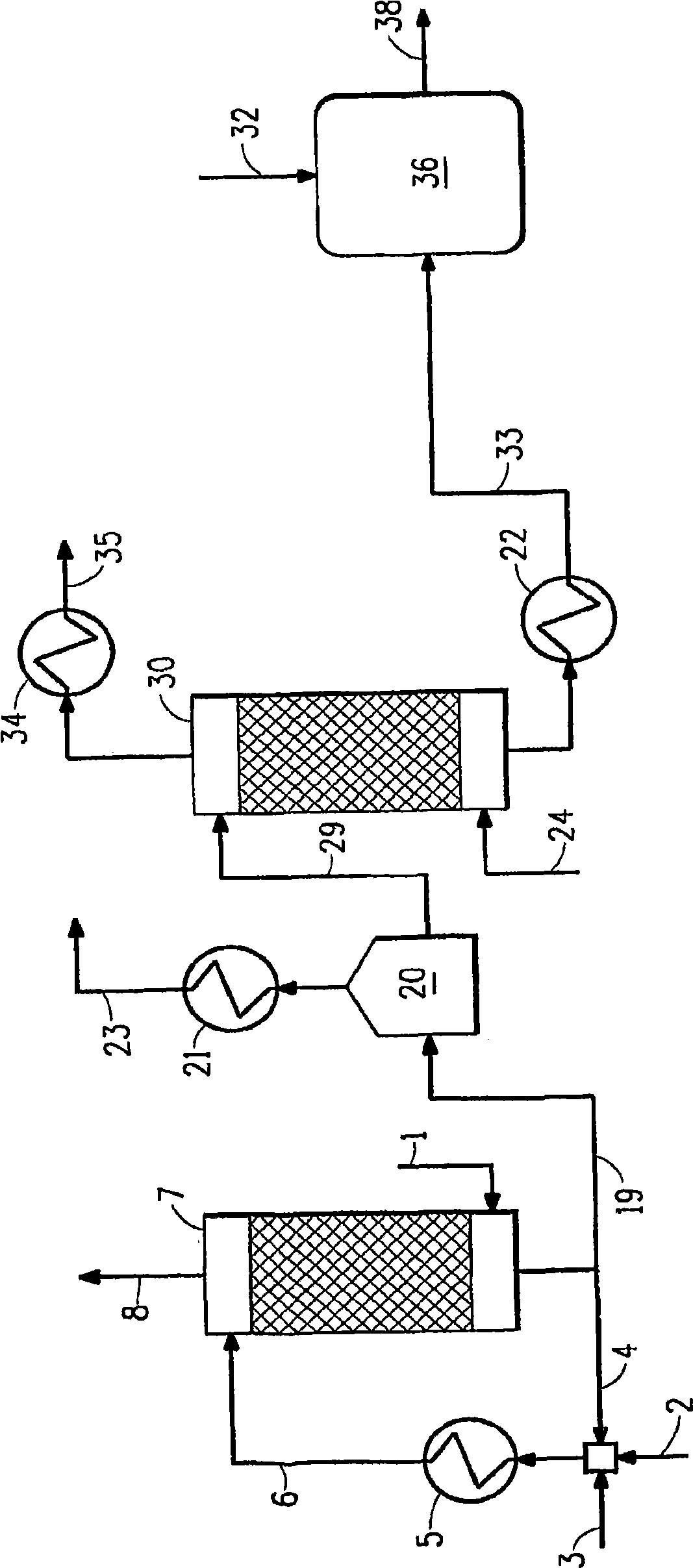
[0069] conduct figure 2 Computer modeling simulation of the method described in . Stripped from 143.3 lb mol / h (65 kg mol / h) of acidic water including 38.6 vol% ammonia (equivalent to 0.28 mass fraction) using recycled acid effluent (weak acid, including 5 wt% sulfuric acid) and fresh acid (98 wt% sulfuric acid) Ammonia scrubbing in tower gas. Flow profiles and concentrations throughout the simulation for this example are shown in Table 2 below.
Embodiment 3
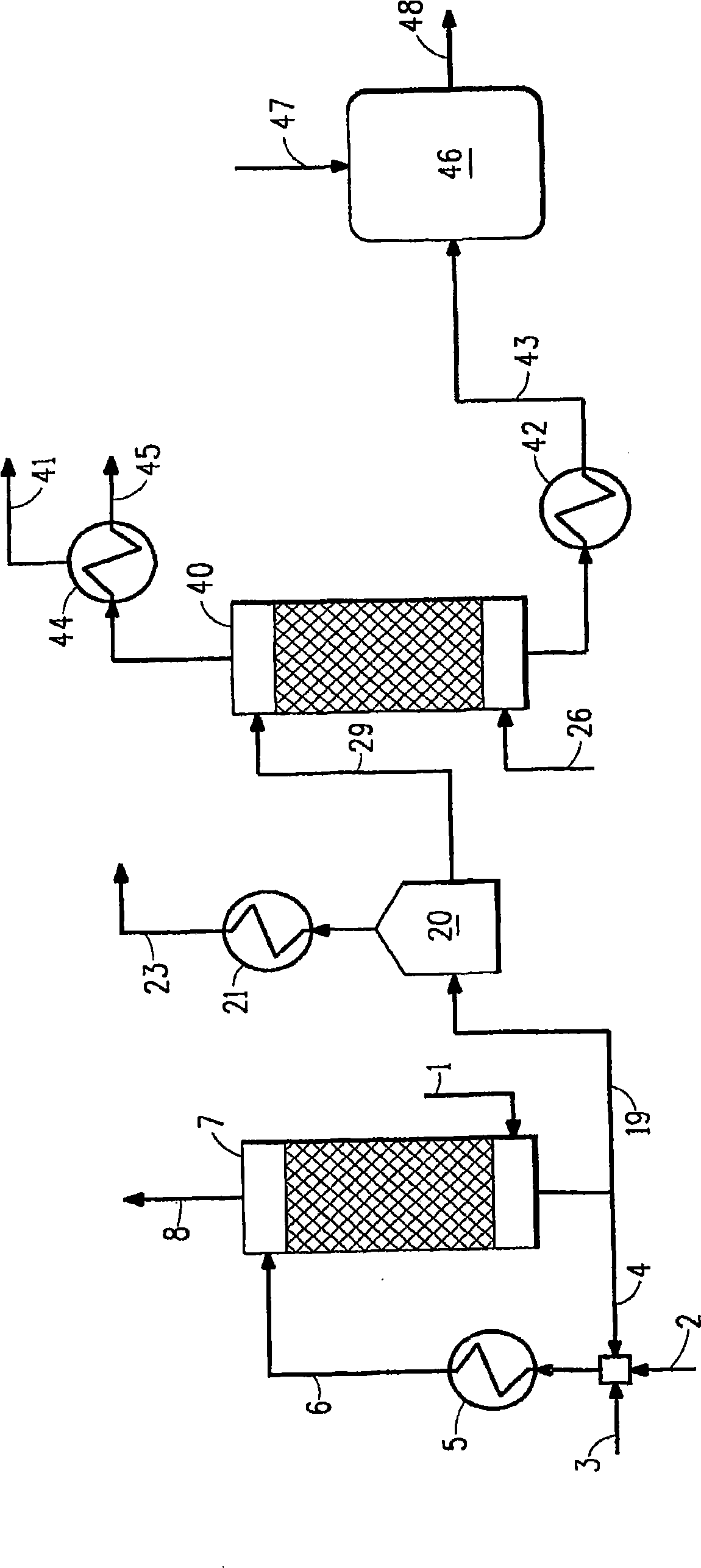
[0071] conduct image 3 Computer modeling simulation of the method described in . Stripped from 143.3 lb mol / h (65 kg mol / h) of acidic water including 38.6 vol% ammonia (equivalent to 0.28 mass fraction) using recycled acid effluent (weak acid, including 5 wt% sulfuric acid) and fresh acid (98 wt% sulfuric acid) Ammonia scrubbing in tower gas. Flow profiles and concentrations throughout the simulation for this example are shown in Table 3 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com