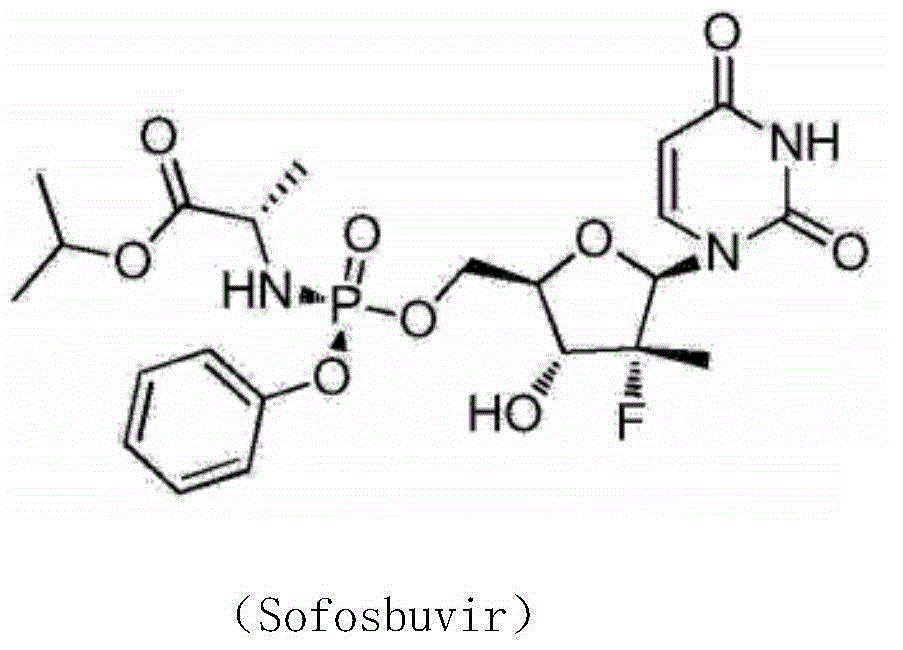
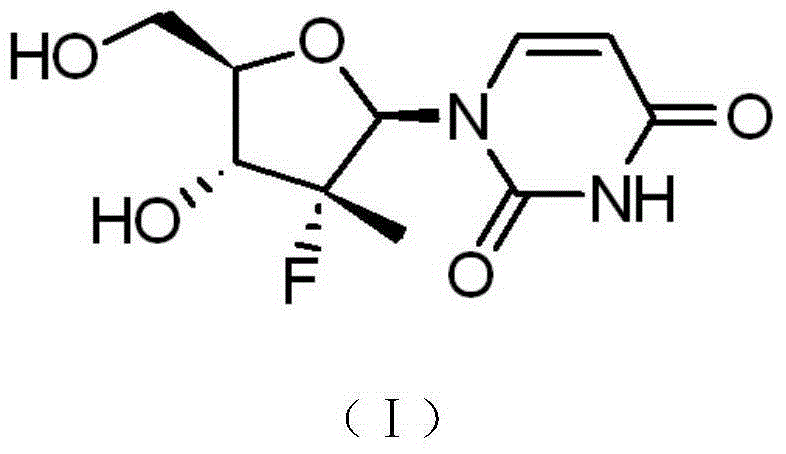
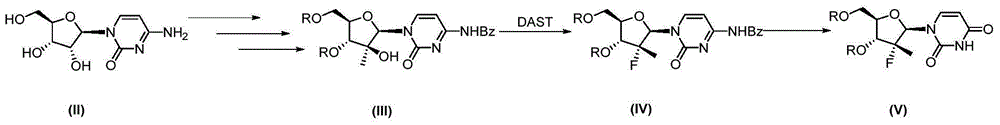
Synthesis method of intermediate compound of sofosbuvir
A synthesis method and intermediate technology, which are applied to the synthesis field of intermediate compounds, can solve the problems of low atom economy, long production cycle, and many three wastes, etc., and achieve the effects of simple and easy synthesis route, low cost and simple steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The synthetic method comprises the following steps:
[0042] (1) Starting with (3R,4R,5R)-3-fluoro-dihydro-4-hydroxy-5-hydroxymethyl-3-methylfuran-2(3H)-one, and pivaloyl chloride A reaction occurs to generate an intermediate product (Ⅻ);
[0043] (2) reduction of intermediate product (XII) to intermediate product (XIII);
[0044] (3) reacting the intermediate product (XIII) with pivaloyl chloride to generate the intermediate product (XIV);
[0045] (4) reacting the intermediate product (XIV) with hydrobromic acetic acid solution to generate the intermediate product (XV);
[0046] (5) performing a Silyl-Hilbert-Johnson reaction on the intermediate product (XV) with uracil to generate the intermediate product (XVI);
[0047] (6) Reacting the intermediate product (XVI) with sodium methoxide to obtain the target product: an intermediate compound of sofosbuvir represented by formula (I).
[0048] In a preferred embodiment, in the synthesis method, the step (1) is: adding ...
Embodiment 1
[0057] The synthesis of embodiment 1 intermediate product (XII)
[0058] Add (3R,4R,5R)-3-fluoro-dihydro-4-hydroxy-5-hydroxymethyl-3-methylfuran-2(3H)-one 200.0g and acetonitrile into a dry 3.0L reaction flask 1.4L. The temperature of the reaction liquid was lowered to 0°C, and 368.0 g of pivaloyl chloride was added dropwise. During the dropwise addition, the temperature was controlled at 0°C. After the dropwise addition, 8.0 g of 4-dimethylaminopyridine was added. Then, the temperature was raised to 10-15° C., and the reaction was stirred for 1 hour. Add 296.0 g of triethylamine dropwise, and control the temperature at 10-15°C during the dropwise addition. After the dropwise addition, the temperature was raised to normal temperature to react for 1 hour. After completion of the reaction, 1000.0 g of ethyl acetate was added, followed by stirring for 30 minutes. After filtering, the filter cake was washed twice with 1000.0 g of ethyl acetate. The two filtrates were combin...
Embodiment 2
[0061] The synthesis of embodiment 2 intermediate product (XIII)
[0062] Under nitrogen protection, 261.0 g of red aluminum and 500.0 g of toluene were added to a dry and anhydrous 1.0 L reaction flask. The temperature was lowered to about -12°C, and 84.0 g of trifluoroethanol was slowly added dropwise. When adding dropwise, control the temperature at about -10°C. After the dropwise addition, the temperature was raised to room temperature and stirred for one hour.
[0063] Under nitrogen protection, 200.0 g of intermediate product (Ⅻ), 400.0 g of toluene and 260.0 g of butyl acetate were added to a dry and anhydrous 3.0 L reaction flask. Lower the temperature to about -12°C, and slowly add the activated red aluminum solution dropwise. During the dropwise addition, the temperature was controlled at about -10°C. After dropping, stir for 15 minutes. HPLC analysis of a sample showed that the reaction was complete. After the reaction was completed, the reaction solution was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com