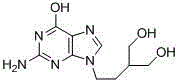
Purification method of penciclovir
A refining method, the technology of penciclovir, which is applied in the field of compound preparation, can solve problems such as unguaranteed, and achieve the effects of low cost, strong repeatability, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
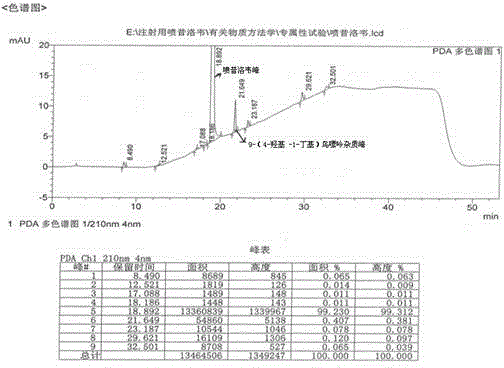
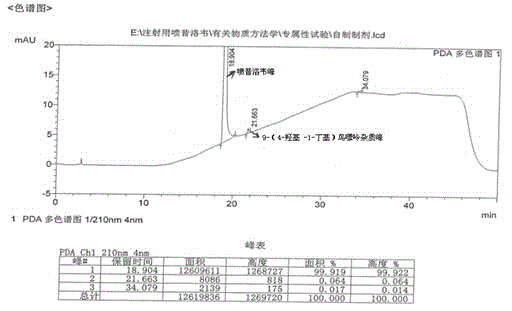
[0024] Under stirring, add 40 g of crude penciclovir with 0.41% impurity content of 9-(4-hydroxy-1-butyl)guanine into 200 g of dimethyl sulfoxide, heat to 60 ° C, keep warm until completely dissolved, and then add 5.5 g aluminum oxide, keep stirring for 1.0 h, filter while it is hot, collect the filtrate and lower the temperature to 15-20°C, add 450 ml of ethanol while stirring, and stir for 2 h to crystallize. Filter, wash the filter cake with a small amount of ethanol, and obtain Penciclovir 37.60g after the filter cake is dried under reduced pressure, wherein 9-(4-hydroxyl-1-butyl)guanine impurity is 0.06%.
Embodiment 2
[0026] Add 40 g of crude penciclovir with 1.40% impurity content of 9-(4-hydroxy-1-butyl) guanine into 320 g of dimethyl sulfoxide under stirring, heat to 60 ° C, keep warm until completely dissolved, and then add 22.4 g aluminum oxide, keep stirring for 1.0 h, filter while it is hot, collect the filtrate and lower the temperature to 15-20°C, add 450 ml of ethanol while stirring, and stir for 2 h to crystallize. Filter, wash the filter cake with a small amount of ethanol, and obtain Penciclovir 37.06g after the filter cake is dried under reduced pressure, wherein 9-(4-hydroxy-1-butyl)guanine impurity is 0.03%.
Embodiment 3
[0028] Under stirring, add 40 g of crude penciclovir with 2.10% impurity content of 9-(4-hydroxy-1-butyl)guanine into 200 g of dimethyl sulfoxide, heat to 60 ° C, keep warm until completely dissolved, and then add 27.0 g aluminum oxide, keep stirring for 0.5h, filter while it is hot, collect the filtrate and lower the temperature to 15-20°C, add 450ml of ethanol while stirring, stir and crystallize for 2h. Filter, wash the filter cake with a small amount of ethanol, and obtain Penciclovir 36.52g after the filter cake is dried under reduced pressure, wherein 9-(4-hydroxyl-1-butyl)guanine impurity is 0.07%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com