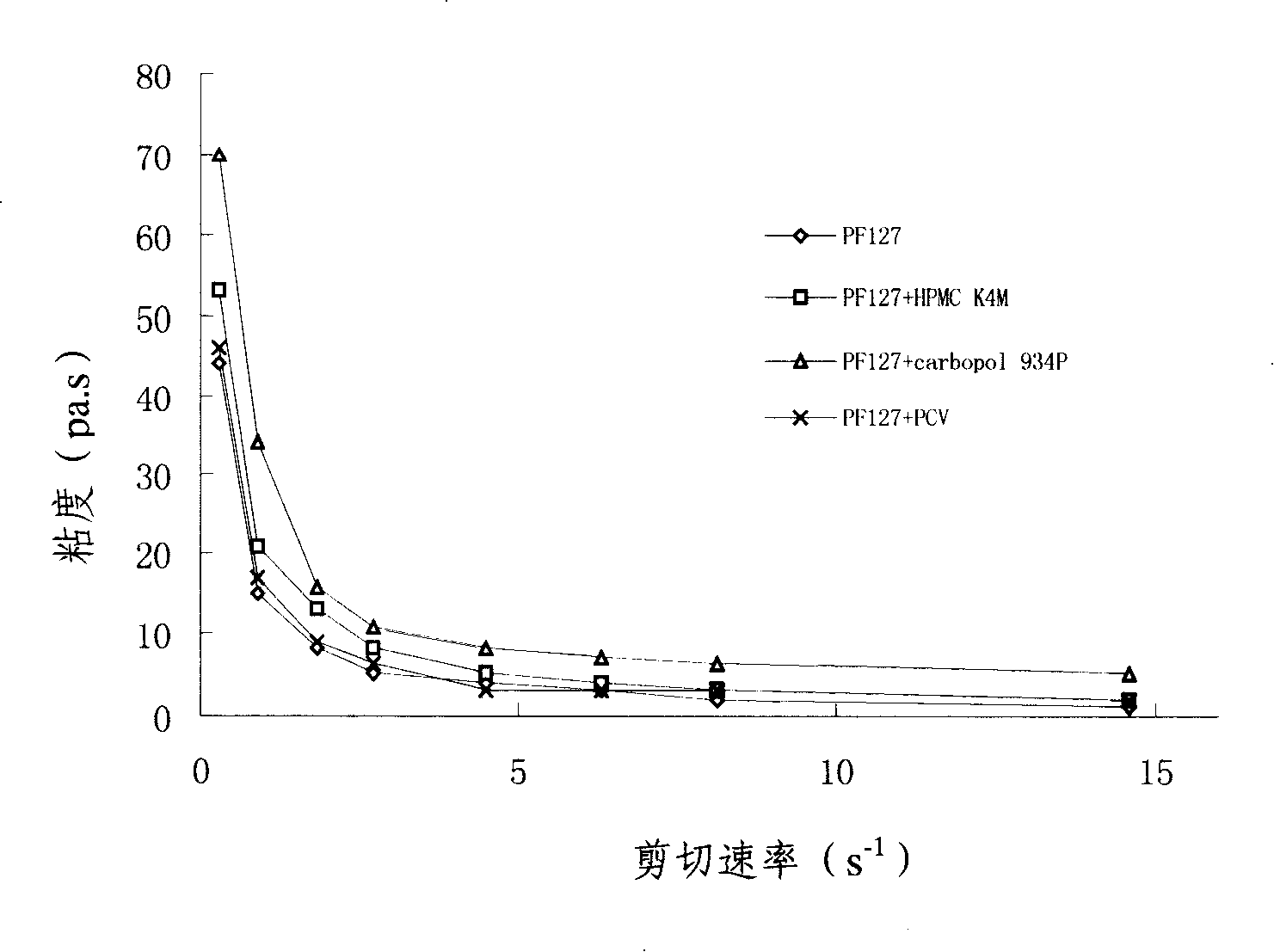
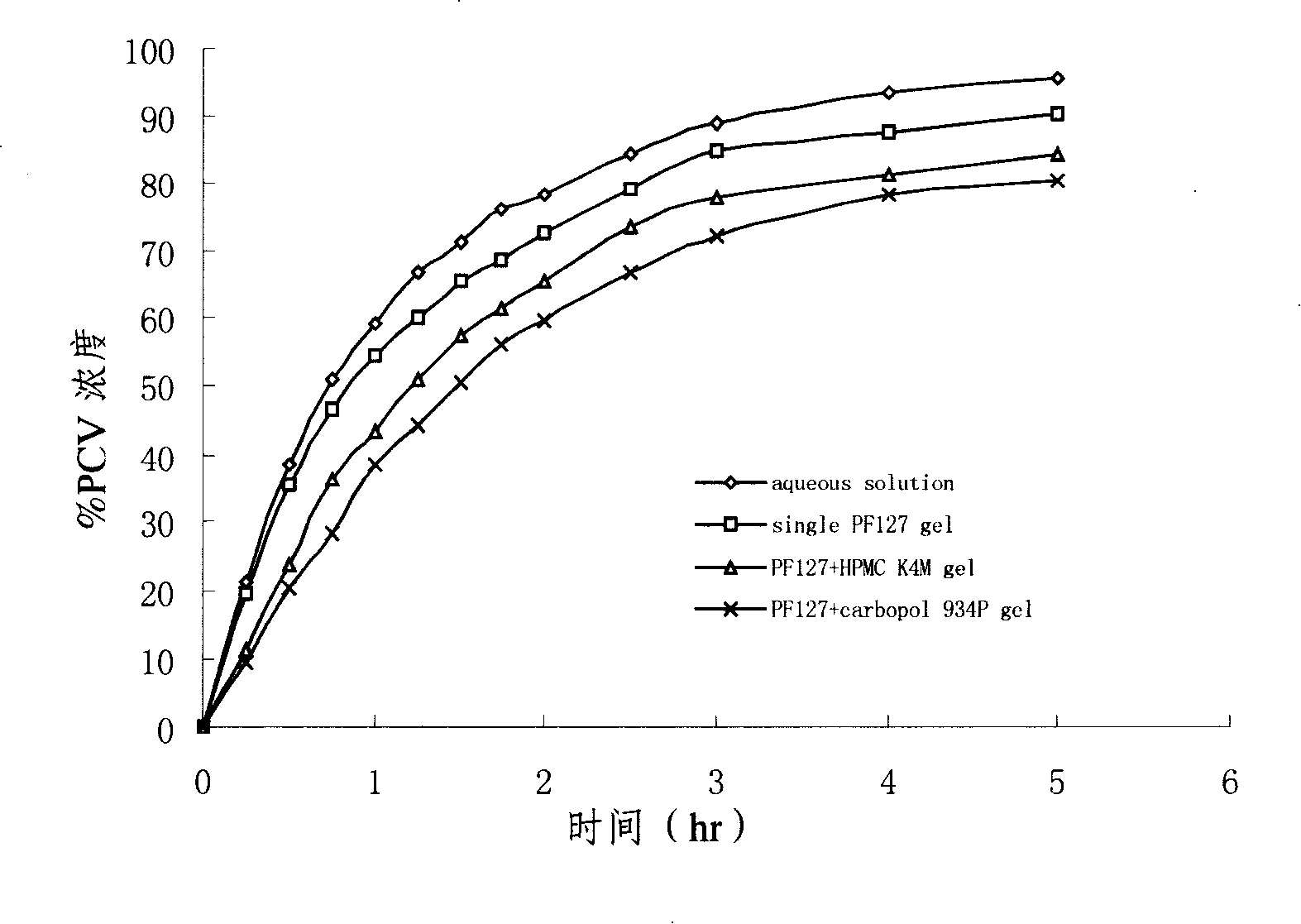
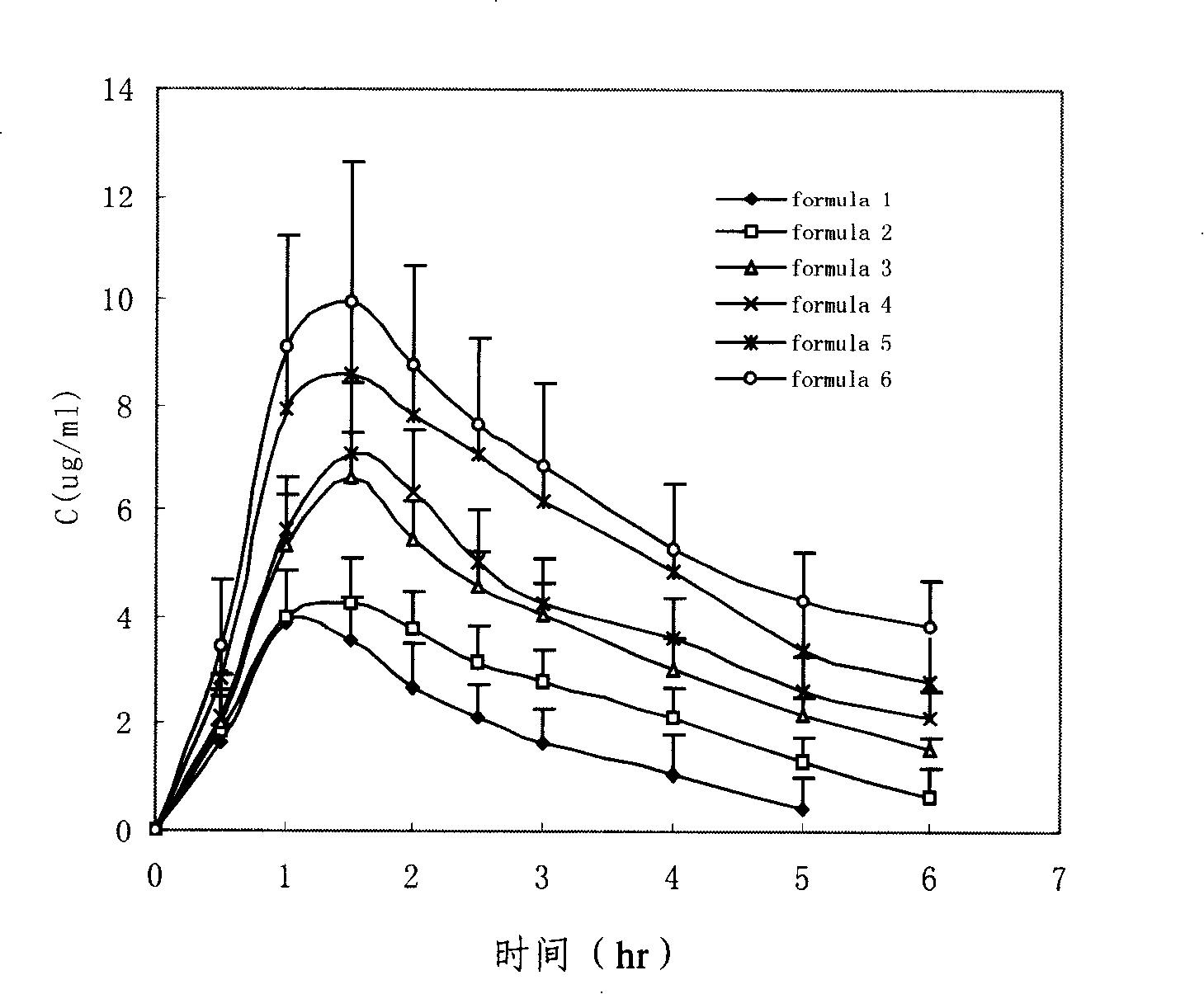
Penciclovir ophthalmic temperature sensitivity in situ gel preparation and preparation method thereof
An in-situ gel, penciclovir technology, applied in the directions of non-active ingredients medical preparations, sensory diseases, antiviral agents, etc., can solve the problem of eye discomfort, unfavorable absorption, short drug retention time and action time and other problems, to achieve the effect of easy and accurate dose control, good biocompatibility, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]PCV ophthalmic in situ gel containing carbomer: Weigh 0.3% carbomer 934P, add distilled water, stir fully, place until completely dissolved, and use it as solution I; weigh 18% PF127, add distilled water, stir to make Partially dissolve, put it in the refrigerator for at least 24 hours until completely dissolved, and use it as solution II; dissolve 0.2% PCV, 5% mannitol, and 0.01% benzalkonium chloride in distilled water, stir to dissolve, and pass through 0.30μm Filter through a microporous membrane, combine the filtrate with solution I and solution II, adjust the pH value to 6.5 with 1mol / L sodium hydroxide solution, add distilled water to the full amount, and stir evenly to obtain the product.
Embodiment 2
[0029] PCV ophthalmic in situ gel containing hydroxypropyl methylcellulose (HPMC): Weigh 0.4% HPMC K4M, add distilled water, heat to 70°C, stir fully, place until completely dissolved, as solution I; weigh Add 18% PF127 into distilled water, stir to partially dissolve, place in the refrigerator for at least 24 hours until completely dissolved, and use it as solution II; dissolve 0.1% PCV, 0.9% sodium chloride, and 0.01% benzalkonium chloride in Stir to dissolve in distilled water, filter through a 0.30 μm microporous membrane, combine the filtrate with solution I and solution II, adjust the pH value to 7.5 with 1mol / L sodium hydroxide solution, add distilled water to the full amount, and stir evenly, that is have to.
Embodiment 3
[0031] PCV ophthalmic in-situ gel containing polyvinylpyrrolidone (PVP): Weigh 20% PF127, add it into distilled water, stir to partially dissolve it, place it in the refrigerator for at least 24 hours until it is completely dissolved, and use it as solution I; Dissolve 0.1% PCV, 0.8% PVP K30, 0.9% sodium chloride, and 0.01% benzalkonium chloride in distilled water, stir to dissolve, filter through a 0.30 μm microporous membrane, combine the filtrate with solution I, and use 1mol / L sodium hydroxide solution to adjust the pH value to 6.5-7.5, add distilled water to the full amount, stir evenly, and get ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com