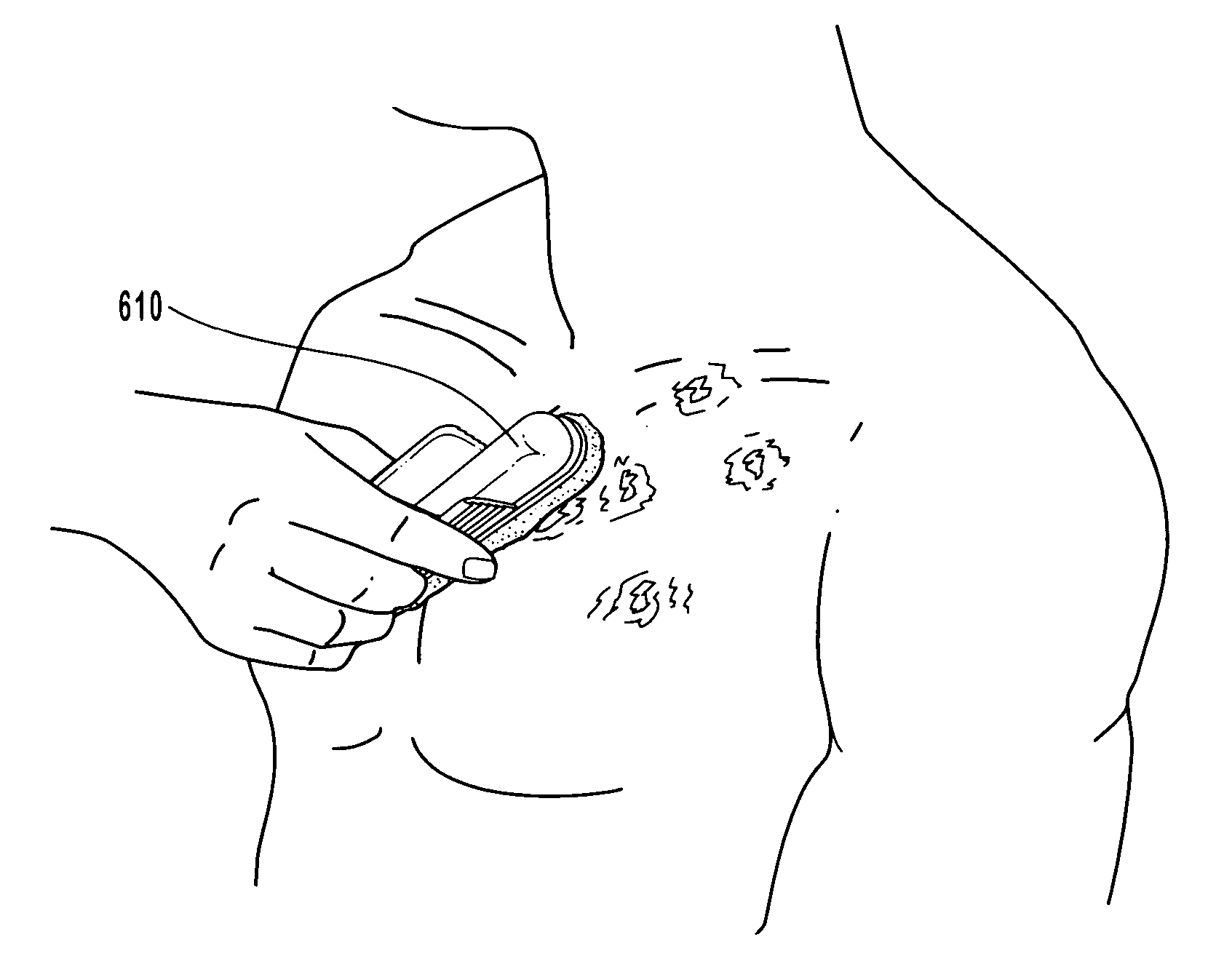
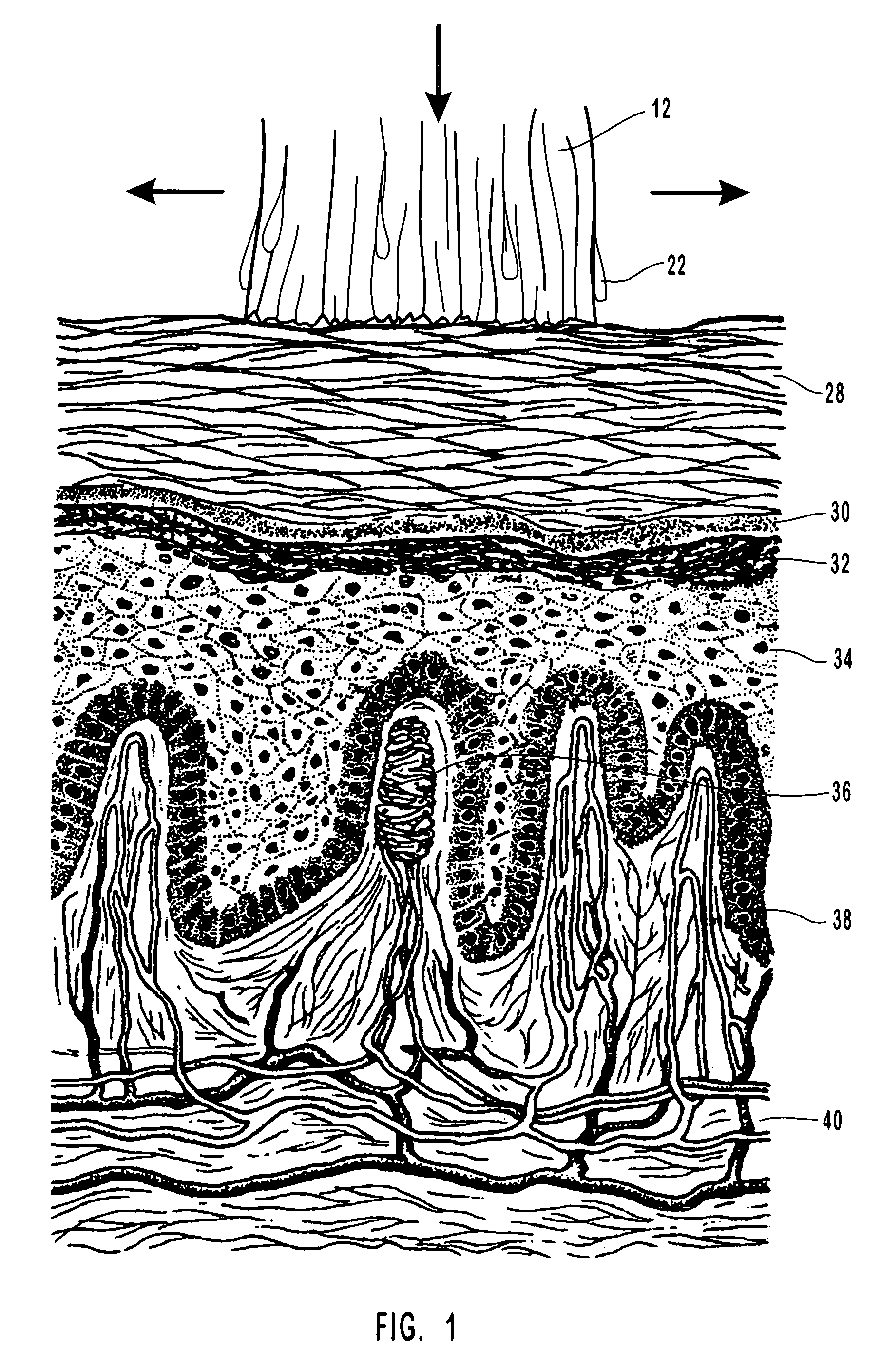
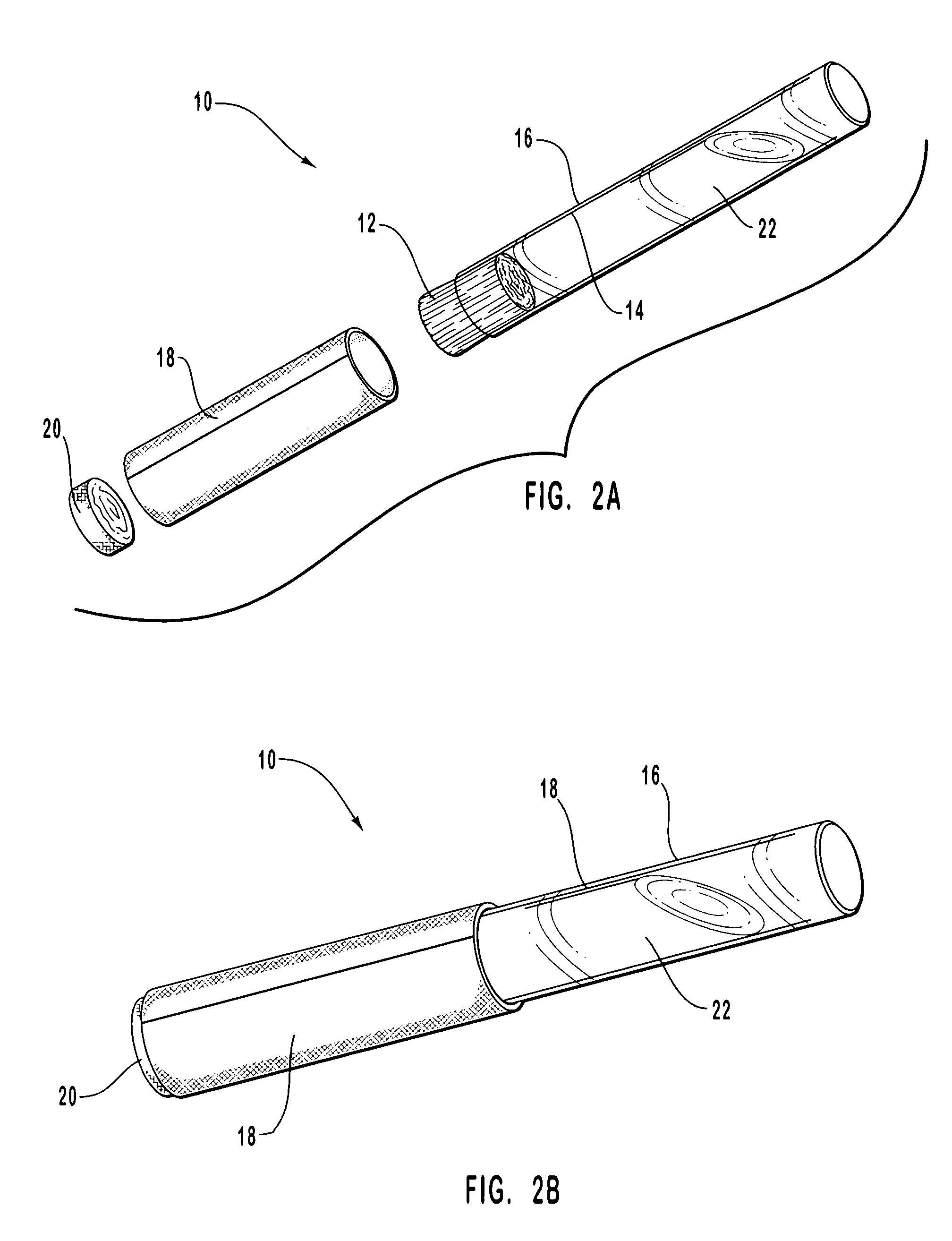
Anti-infective compositions, methods and systems for treating pathogen-induced disordered tissues
a composition and antiviral technology, applied in the field of pathogen-induced disordered tissue treatment, can solve the problems of trigeminal nerve, difficult to treat, difficult to swallow, eat and facial swelling, etc., and achieve the effect of stimulating rapid immunological attack, effective treatment methods, and extraordinary therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0376] In a first example, disordered tissue that has a redness of 10 of a nominal red scale is subjected to the inventive method by impregnating an applicator with about 0.02% benzalkonium chloride in isopropyl alcohol composition. The impregnated applicator is then vigorously applied to a labial disordered tissue for a time period of about 30 seconds. During the application time period, about 0.2 ml of the inventive composition is absorbed into the patient's disordered tissue. The patient's disordered tissue is estimated to have an area of about 0.5 cm2. The patient's disordered tissue is then examined and is found to have a decreased nominal red scale to about 6 after about 24 hours and an increased eosinophil assay of about 40% before about one hour.
example 2
[0379] In a second example, all conditions are the same as in the first example with the following variations. An embodiment of the inventive composition is applied to a typical sterile bandage and left over the patient's disordered tissue for about one hour. The sterile bandage may double as part of the applicator. The composition contains, in addition to about 0.02% benzalkonium chloride in isopropyl alcohol, about 5% of a composition of lidocaine and prilocalne in about a 1:1 mixture. After the one hour time period, the patient's skin is substantially numbed, and the applicator is vigorously rubbed into the disordered tissue for about 30 seconds. The patient experiences significantly less pain than that experienced in the first example. The patient's disordered tissue is then examined and is found to have a decreased nominal red scale to about 3 from a beginning of eight after about 24 hours and an increased eosinophil assay of about 50% before about one hour.
example 3
[0382] In a third example, a patient with pink eye is administered the inventive composition containing about 0.01% benzalkonium chloride in a carrier that is substantially nonirritating to the sclera and supporting eye tissue. The patient, with washed and disinfected hands, then rubs the closed eyelid with the hand or fingers for about 30 seconds. The patient's eye is then examined and is found to have a decreased nominal red scale to about 1 after about 24 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| period of time | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com