Penciclovir freeze dried and method of manufacturing the same
A technology of freeze-dried powder injection and penciclovir is applied in the field of medicine to achieve the effects of solving poor bioavailability, good finished product molding, and ensuring safety and convenience.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
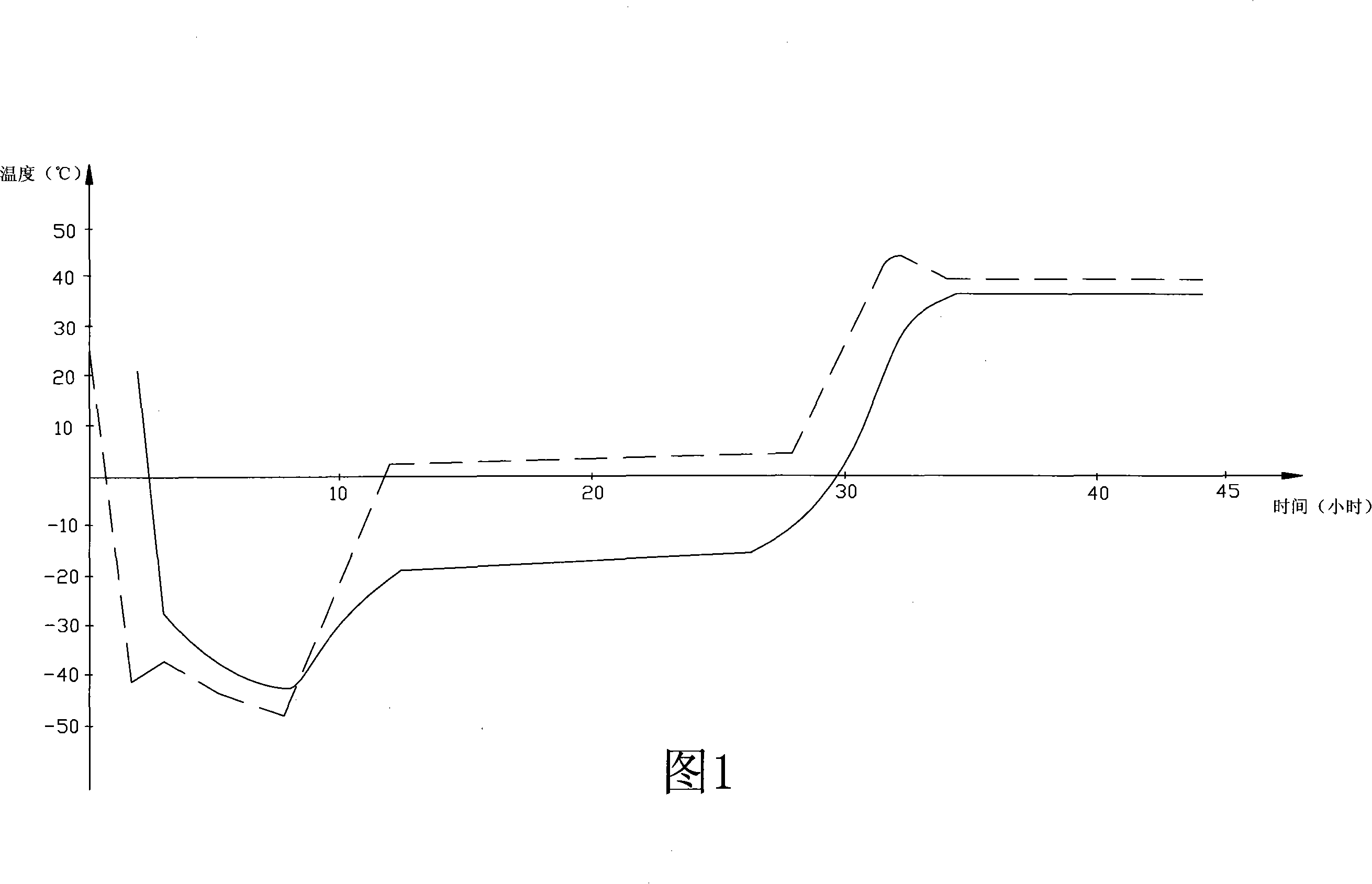
[0027] Get 250g of penciclovir, dissolve with 5% sodium hydroxide solution until clarification (control pH value 11.0); 100g of low-molecular-weight d-glucoside is prepared into a 10% solution with water for injection, add 0.5% activated carbon, and boil for 30min , cooling and filtering. Mix the above two solutions, add 0.5% activated carbon, stir for 30 minutes, filter, take a sample to measure the content, add water for injection to the specified volume, and measure the pH value. Put the packaged products into the freeze dryer for freeze-drying (the freeze-drying curve is shown in Figure 1), pre-freeze for 3 hours after entering the box, the shelf temperature is -40°C, the product temperature is -25°C, and the vacuum pump is turned on. Sublimation begins, and the temperature of the product during the sublimation stage is controlled at -18°C (the eutectic point of the product is about -9°C). After the water line reaches the bottom, the temperature of the product slowly rises...
Embodiment 2
[0029] Get 300g of penciclovir, dissolve with 5% sodium hydroxide solution to clarification (control pH value 11.0); 120g of low-molecular-weight d-glucoside, first prepare 10% solution with water for injection, add 0.5% active carbon, boil for 30min , cooling and filtering. Mix the above two solutions, add 0.5% activated carbon, stir for 30 minutes, filter, take a sample to measure the content, add water for injection to the specified volume, and measure the pH value. Put the packaged products into the freeze dryer for freeze-drying, and pre-freeze for 4 hours after entering the box. The temperature of the shelf is -45°C, and the temperature of the product is -25°C. Turn on the vacuum pump and the product starts to sublimate. 20°C (the eutectic point of the product is about -9°C), after the water line reaches the bottom, the temperature of the product slowly rises to about 37°C, the temperature of the shelf is 40°C, keep it warm for 10 hours, and press the plug out of the box...
Embodiment 3
[0031] Get 400g of penciclovir, dissolve with 5% sodium hydroxide solution until clarification (controlling pH value 11.0); 160g of low-molecular-weight d-glucoside is prepared into a 10% solution with water for injection, add 0.5% activated carbon, and boil for 30min , cooling and filtering. Mix the above two solutions, add 0.5% activated carbon, stir for 30 minutes, filter, take a sample to measure the content, add water for injection to the specified volume, and measure the pH value. Put the packaged products into the freeze dryer for freeze-drying, and pre-freeze for 5 hours after entering the box. The shelf temperature is -50°C, the product temperature is -30°C, and the vacuum pump is turned on. The product starts to sublime, and the product temperature is controlled at - 20°C (the eutectic point of the product is about -9°C), after the water line reaches the bottom, the temperature of the product slowly rises to about 37°C, the temperature of the shelf is 41°C, keep it w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com