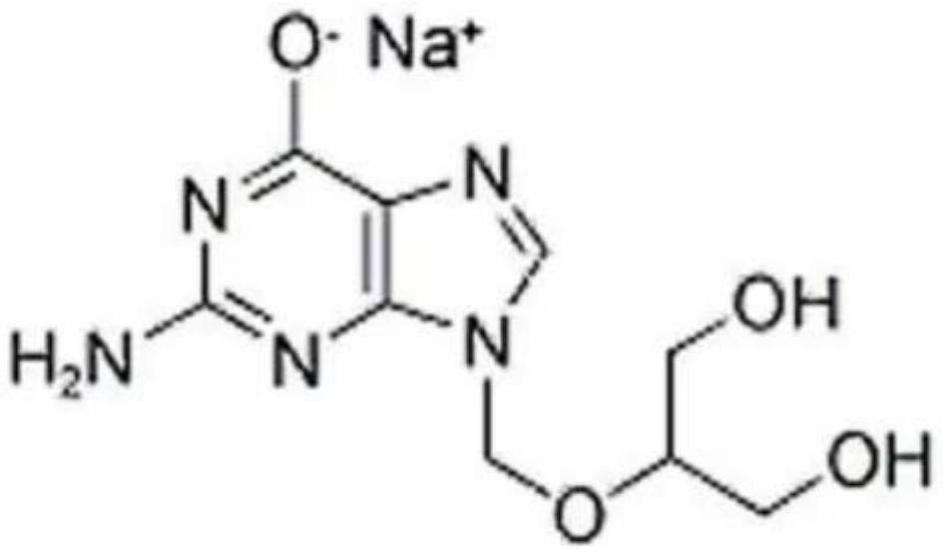
A kind of preparation method of ganciclovir sodium freeze-dried powder for injection
A technology of ganciclovir sodium and freeze-dried powder, which is applied in the field of medicine, can solve the problems of hygroscopicity and purity of ganciclovir or ganciclovir sodium freeze-dried powder, and achieve improved appearance and moisture absorption. Sexually low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
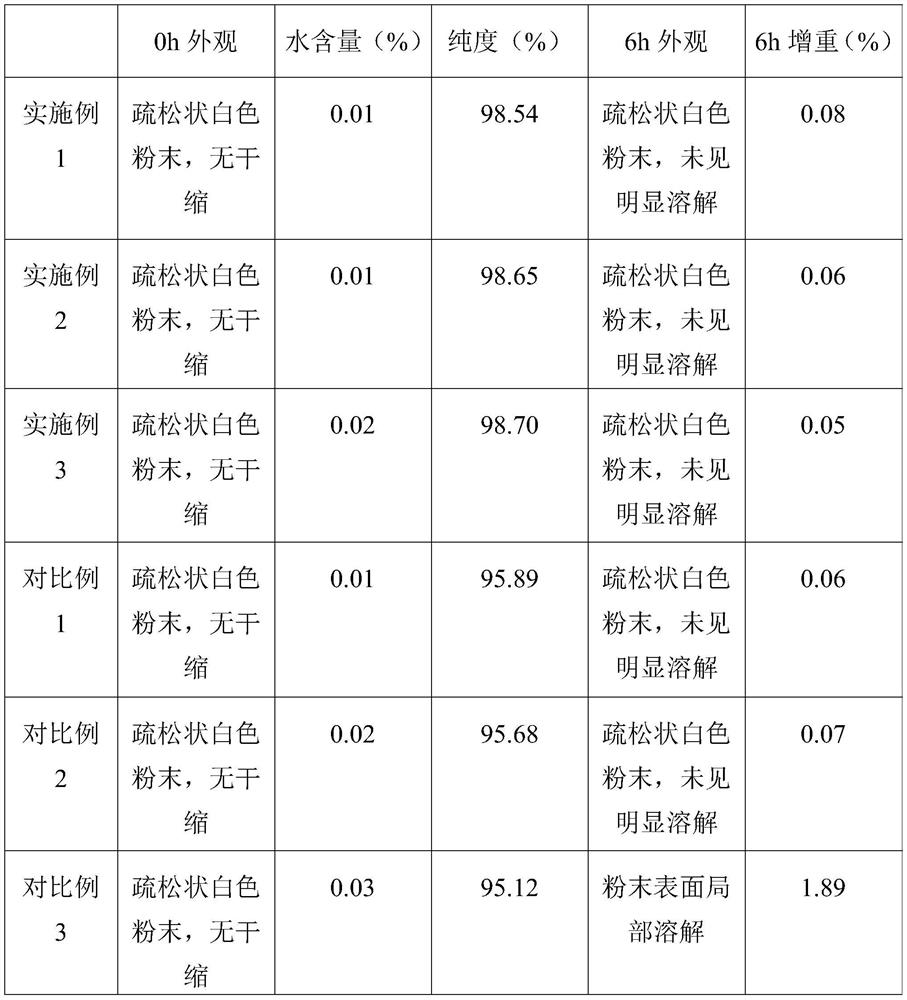
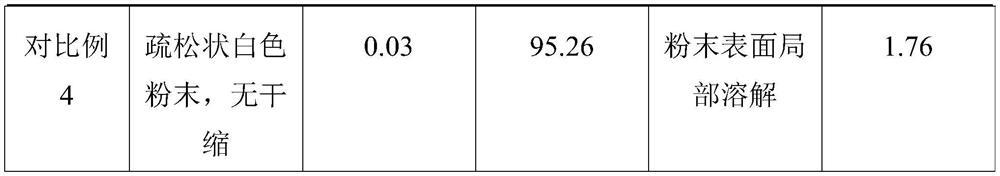
Embodiment 1
[0025] A preparation method of ganciclovir sodium freeze-dried powder for injection, comprising the following steps in turn:
[0026] (1) Add mannitol to water for injection to prepare mannitol solution. The mass concentration of the mannitol solution is 5%.
[0027] (2) adding activated carbon to the mannitol solution of step (1), stirring for 20min, standing for 15min, and then coarsely filtering and decarbonizing; passing the mannitol solution after coarsely filtering and decarbonizing through a 0.22 μm sterile mixed cellulose film to prepare A purified mannitol solution was obtained. The mass concentration of the activated carbon in the mannitol solution is 0.05%. The weight ratio of the ganciclovir sodium to the mannitol solution is 0.010:1.
[0028] (3) adding ganciclovir sodium to the purified mannitol solution, and then passing a 0.22 μm sterile mixed cellulose film to prepare a ganciclovir sodium solution; the temperature of the ganciclovir sodium solution is contr...
Embodiment 2
[0032] A preparation method of ganciclovir sodium freeze-dried powder for injection, comprising the following steps in turn:
[0033] (1) Add mannitol to water for injection to prepare mannitol solution. The mass concentration of the mannitol solution is 2%.
[0034] (2) adding activated carbon to the mannitol solution of step (1), stirring for 30min, standing for 20min, and then coarsely filtering and decarbonizing; passing the mannitol solution after coarsely filtering and decarbonizing through a 0.22 μm sterile mixed cellulose film to prepare A purified mannitol solution was obtained. The mass concentration of the activated carbon in the mannitol solution is 0.1%. The weight ratio of the ganciclovir sodium to the mannitol solution is 0.020:1.
[0035] (3) adding ganciclovir sodium to the purified mannitol solution, and then passing a 0.22 μm sterile mixed cellulose film to prepare a ganciclovir sodium solution; the temperature of the ganciclovir sodium solution is contro...
Embodiment 3
[0039] A preparation method of ganciclovir sodium freeze-dried powder for injection, comprising the following steps in turn:
[0040] (1) Add mannitol to water for injection to prepare mannitol solution. The mass concentration of the mannitol solution is 4%.
[0041] (2) adding activated carbon to the mannitol solution in step (1), stirring for 25 minutes, leaving it to stand for 18 minutes, and then coarsely filtering and decarbonizing; passing the mannitol solution after coarse filtering and decarbonizing through a 0.22 μm sterile mixed cellulose film to prepare A purified mannitol solution was obtained. The mass concentration of the activated carbon in the mannitol solution is 0.07%. The weight ratio of the ganciclovir sodium to the mannitol solution is 0.015:1.
[0042] (3) adding ganciclovir sodium to the purified mannitol solution, and then passing a 0.22 μm sterile mixed cellulose film to prepare a ganciclovir sodium solution; the temperature of the ganciclovir sodiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com