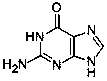
Method for determining content of guanine in ganciclovir
A determination method, guanine technology, applied in measuring devices, instruments, scientific instruments, etc., can solve the problem of incomplete separation of ganciclovir and guanine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: one of guanine content assay methods in ganciclovir:
[0028] 1. Experimental equipment and chromatographic conditions
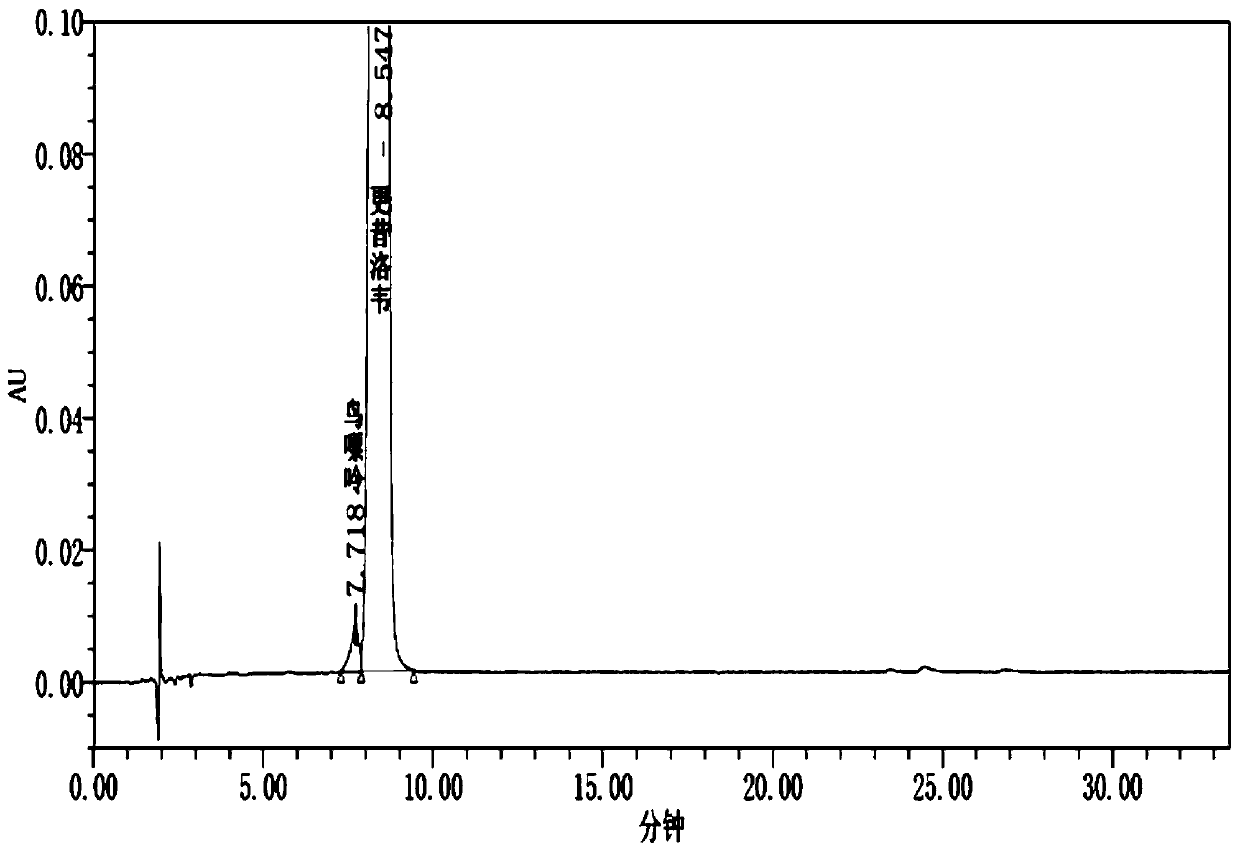
[0029] Shimadzu liquid chromatograph, CHIRALCEL OJ-H chromatographic column (4.6*200mm, 5μm), with n-hexane-isopropanol (80:20) as mobile phase, isocratic elution, flow rate 1.0ml / min, The column temperature is 30° C., the measurement wavelength is 252 nm, and the injection volume is 10 μl.
[0030] 2. Determination of samples
[0031] Accurately weigh an appropriate amount of guanine reference substance, and use mobile phase to prepare a solution containing 5 μg of guanine per 1 ml as the reference solution; accurately weigh an appropriate amount of ganciclovir, and use mobile phase to prepare a solution containing ganciclovir per 1 ml Wei 5mg solution, as the test solution. According to the chromatographic conditions under the guanine content determination item in ganciclovir, each 10 μ l of need testing solution and reference solut...
Embodiment 2
[0032] Embodiment 2: two of guanine content assay methods in ganciclovir:
[0033] 1. Experimental equipment and chromatographic conditions
[0034] Shimadzu liquid chromatograph, CHIRALCEL OJ-H chromatographic column (4.6*200mm, 5μm), using n-hexane-isopropanol (90:10) as mobile phase, for isocratic elution, flow rate 1ml / min, column The temperature is 30°C, the measurement wavelength is 252nm, and the injection volume is 10μl.
[0035] 2. Determination of samples
[0036] Accurately weigh an appropriate amount of guanine reference substance, and use mobile phase to prepare a solution containing 5 μg of guanine per 1 ml as the reference solution; accurately weigh an appropriate amount of ganciclovir, and use mobile phase to prepare a solution containing ganciclox per 1 ml Wei 5mg solution, as the test solution. According to the chromatographic conditions under the guanine content determination item in ganciclovir, each 10 μ l of need testing solution and reference solution...
Embodiment 3
[0037] Embodiment 3: three of guanine content assay methods in ganciclovir:
[0038] 1. Experimental equipment and chromatographic conditions
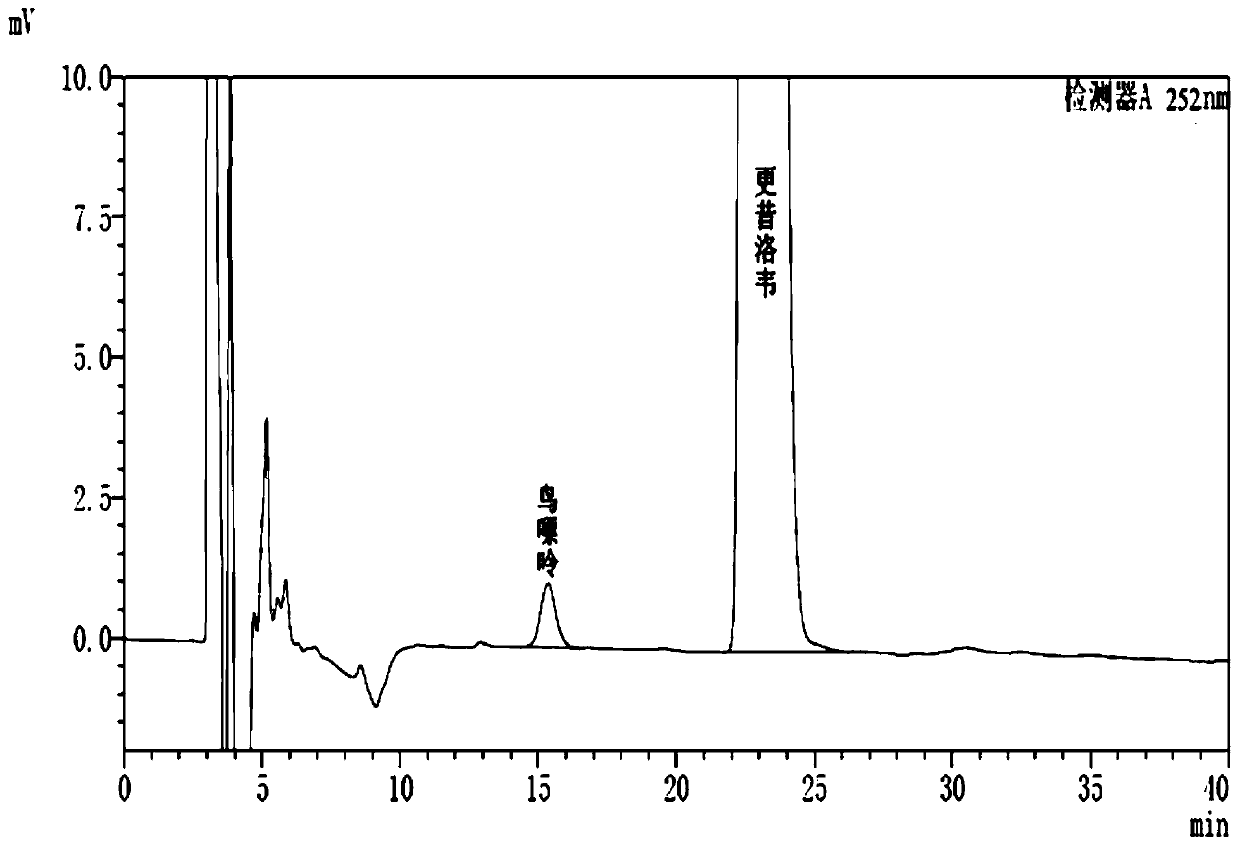
[0039] Shimadzu liquid chromatograph, CHIRALCEL OJ-H chromatographic column (4.6*200mm, 5μm), with n-hexane-isopropanol (70:30) as mobile phase, isocratic elution, flow rate 1.0ml / min, The column temperature is 30° C., the measurement wavelength is 252 nm, and the injection volume is 10 μl.
[0040] 2. Determination of samples
[0041] Accurately weigh an appropriate amount of guanine reference substance, and use mobile phase to prepare a solution containing 5 μg of guanine per 1 ml as the reference solution; accurately weigh an appropriate amount of ganciclovir, and use mobile phase to prepare a solution containing ganciclox per 1 ml Wei 5mg solution, as the test solution. According to the chromatographic conditions under the guanine content determination item in ganciclovir, each 10 μ l of the need testing solution and the referen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com