Preparation method and application of ganciclovir
A technology of ganciclovir and hydrolysis reaction, which is applied in the field of drug synthesis, can solve the problems of too many solvents, difficulty in completing the reaction, and unfavorable industrial production, so as to reduce the amount of solvent used, reduce the occurrence of side reactions, and improve the utilization of raw materials rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
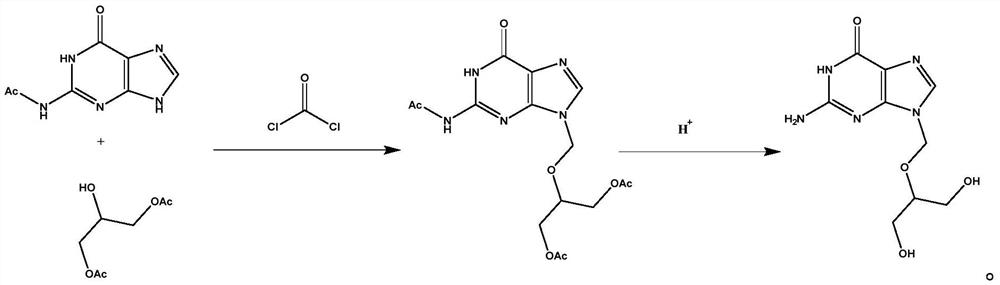
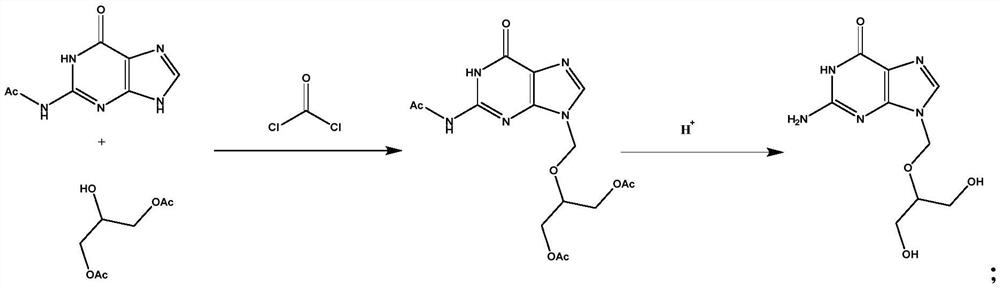
[0031] Embodiment 1 A kind of preparation method of Ganciclovir A1
[0032] This embodiment provides a preparation method of ganciclovir A1, the specific preparation method is to weigh 19.6g of chloroformyl chloride and add it to 100ml of DMF for dissolving and stirring, and weigh 38.6g of N-(6-carbonyl-6, 9-dihydro-1H-purin-2-yl)acetamide and 35.2g 2-hydroxyl-1,3-propylene glycol diacetate were added to the DMF solution of chloroformyl chloride under continuous stirring to dissolve simultaneously, and then weighed respectively Take 42mlTEA and 76g HATU, raise the temperature to 110°C and keep stirring for 2h, monitor the reaction process by TLC, the disappearance of the raw material point is the end point of the reaction, slowly cool down to room temperature, add 200ml ethyl acetate and 50ml saturated saline, stir for 0.5h, then statically Separate the liquid and concentrate the organic phase until the ethyl acetate is completely evaporated to obtain the crude product of tria...
Embodiment 2~6
[0037] The preparation method of embodiment 2~6 ganciclovir A2~A6
[0038] The preparation methods of ganciclovir A2-A6 provided in Examples 2-6 are basically the same as those in Example 1, the difference is only in some process parameters, and the specific process parameters are shown in Table 1.
[0039] Table 1: Preparation process parameters table of Ganciclovir A2~A6
[0040]
[0041]
[0042] All the other parameters are the same as in Example 1.
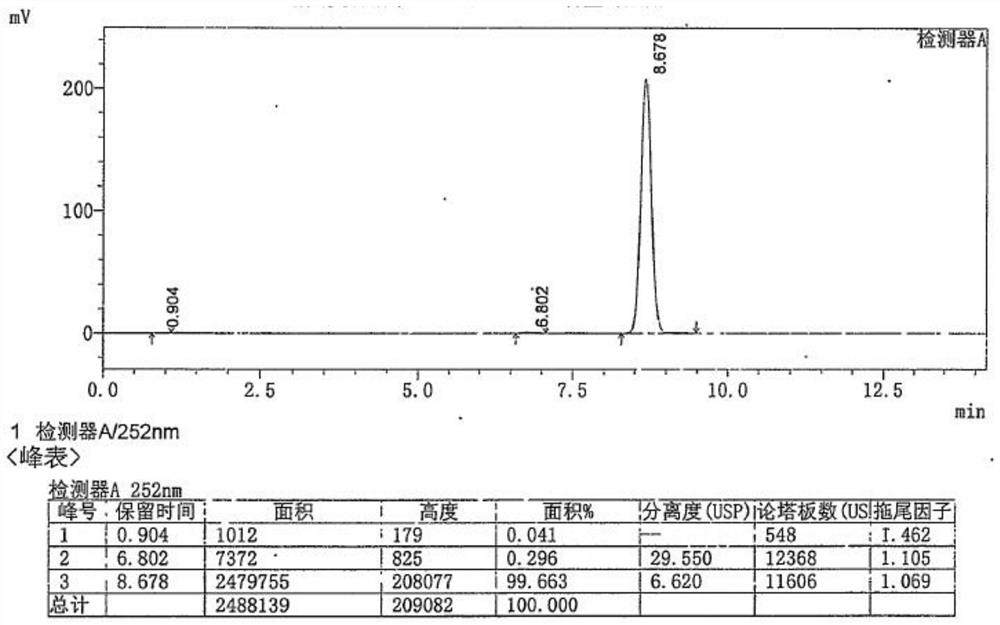
[0043] The characterization data of the products Ganciclovir A1-A6 obtained in Examples 1-6 are consistent with the reports in the literature (J.C.Martin, C.A.Dvorak, D.F.Smee, T.R.Matthews, J.P.H.Verheyden, J.Med.Chem., 1983,26,759-761) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com