Ganciclovir injection and preparation process thereof
A ganciclovir, preparation technology, applied in the field of ganciclovir injection and its preparation, can solve the problems of solubility increase, glass flake dissolution, poor solubility, etc., and achieve good compatibility, visible foreign matter and insoluble particles Falling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] prescription:
[0015]
[0016] crafting process:
[0017] The preparation method is:
[0018] (1) Add 1 / 10 of the amount of ganciclovir sodium hydroxide under stirring with water for injection, stir to dissolve, slowly pour the prescription amount of ganciclovir, and stir to completely dissolve. Adjust the pH to 11.2 with sodium hydroxide.
[0019] (2) Add 0.3% (w / v) activated carbon and stir for 10 minutes, without adding water for injection to the full amount, filter and circulate for 15 minutes, sample and test the content and pH value after passing, then use three-in-one equipment to make plastic PP ampoules and fill ,seal. Sterilize at 115°C for 30 minutes. Pack and inspect the lamp after passing the inspection.
Embodiment 2
[0021] prescription:
[0022]
[0023] The preparation method is:
[0024] (1) Add the prescription amount of sodium hydroxide under stirring with water for injection, stir to dissolve, slowly pour the prescription amount of ganciclovir, and stir to dissolve completely. Adjust the pH to 11.2 with sodium carbonate.
[0025] (2) Add 0.3% (w / v) activated carbon and stir for 10 minutes, without adding water for injection to the full amount, filter and circulate for 15 minutes, sample and test the content and pH value after passing, then use three-in-one equipment to make plastic PP ampoules and fill ,seal. Sterilize at 115°C for 30 minutes. Pack and inspect the lamp after passing the inspection.
Embodiment 3
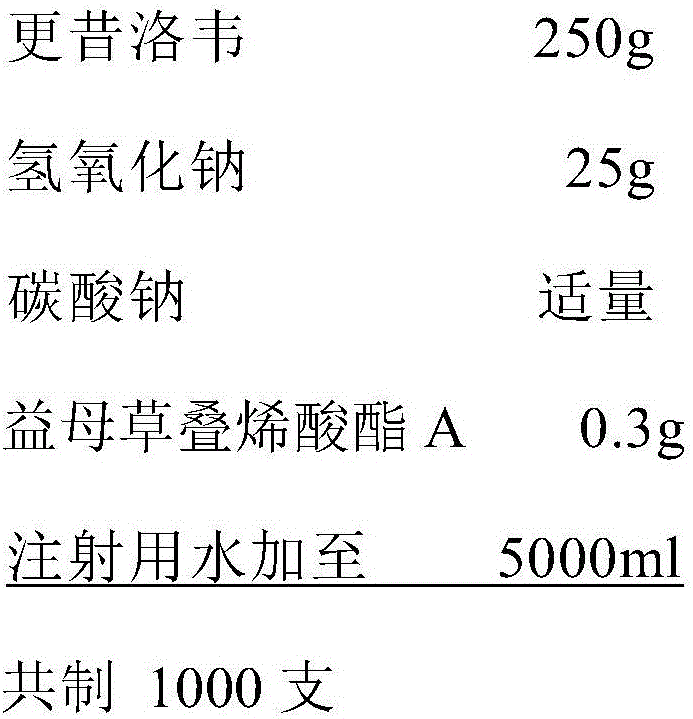
[0027] prescription:
[0028]
[0029] The preparation method is:
[0030] (1) Add the prescription amount of sodium hydroxide under stirring with water for injection, stir to dissolve, slowly pour the prescription amount of ganciclovir and motherwort A, and stir to dissolve completely. Adjust the pH to 11.2 with sodium carbonate.
[0031] (2) Add 0.3% (w / v) activated carbon and stir for 10 minutes, without adding water for injection to the full amount, filter and circulate for 15 minutes, sample and test the content and pH value after passing, then use three-in-one equipment to make plastic PP ampoules and fill ,seal. Sterilize at 115°C for 30 minutes. Pack and inspect the lamp after passing the inspection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com