Ganciclovir composition for injection and freeze drying process of ganciclovir composition
A technology of ganciclovir and drying process, applied in the field of ganciclovir composition for injection and its freeze-drying process, can solve the problems such as difficult removal of water, easy collapse and shrinkage of structure, prolonged freeze-drying cycle, etc. It is not easy to remove, the prescription is simple and reasonable, and the effect of prolonging the freeze-drying cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
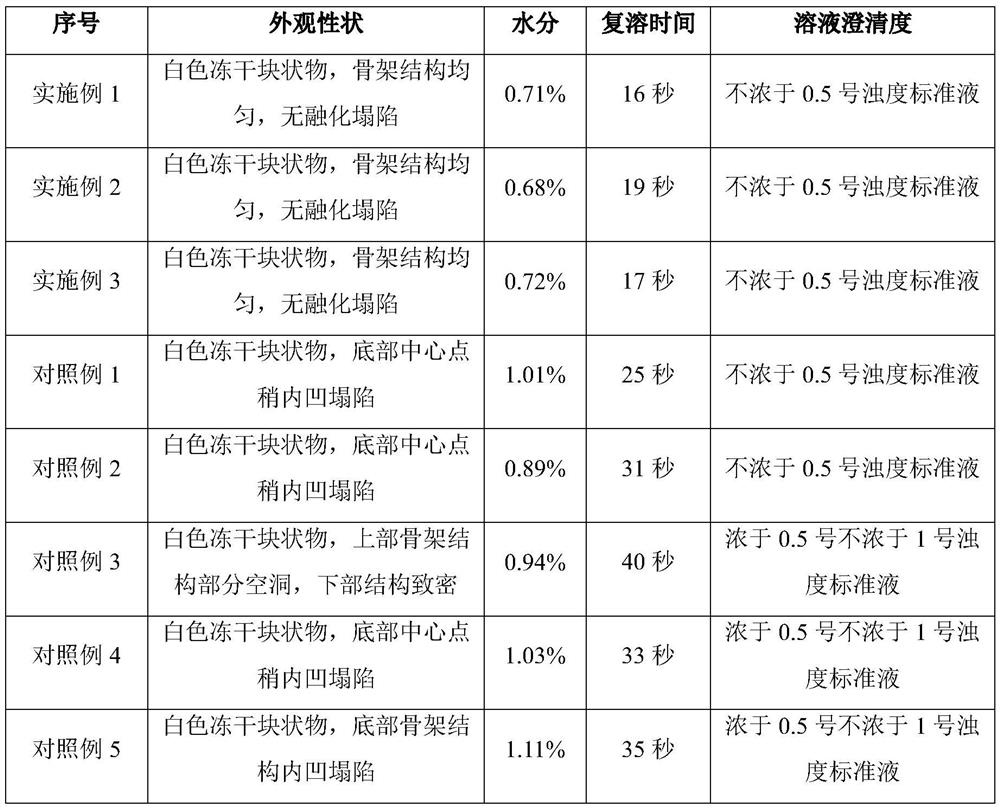
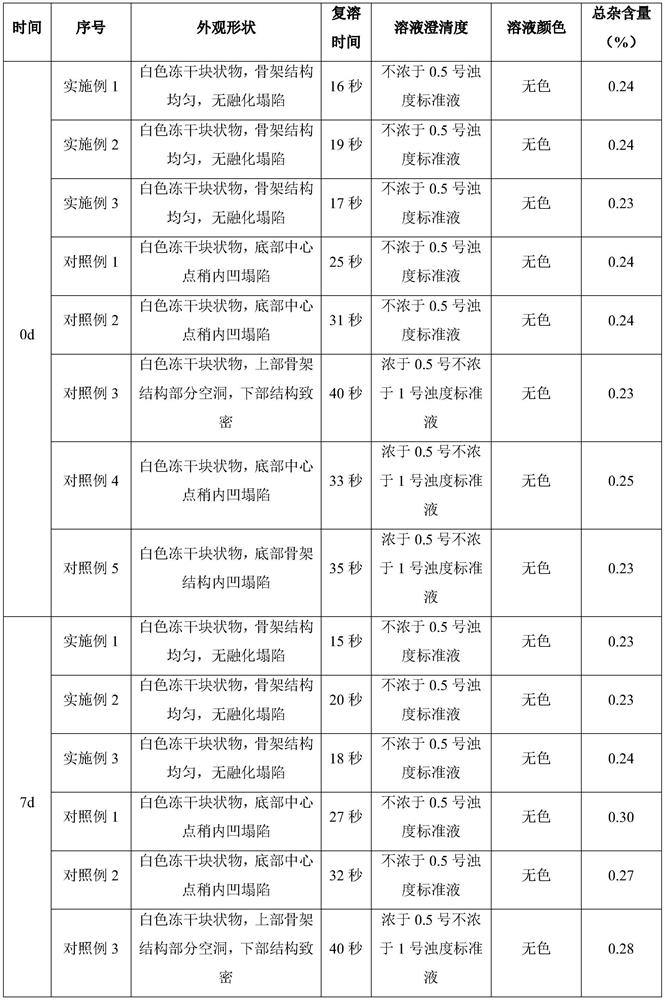
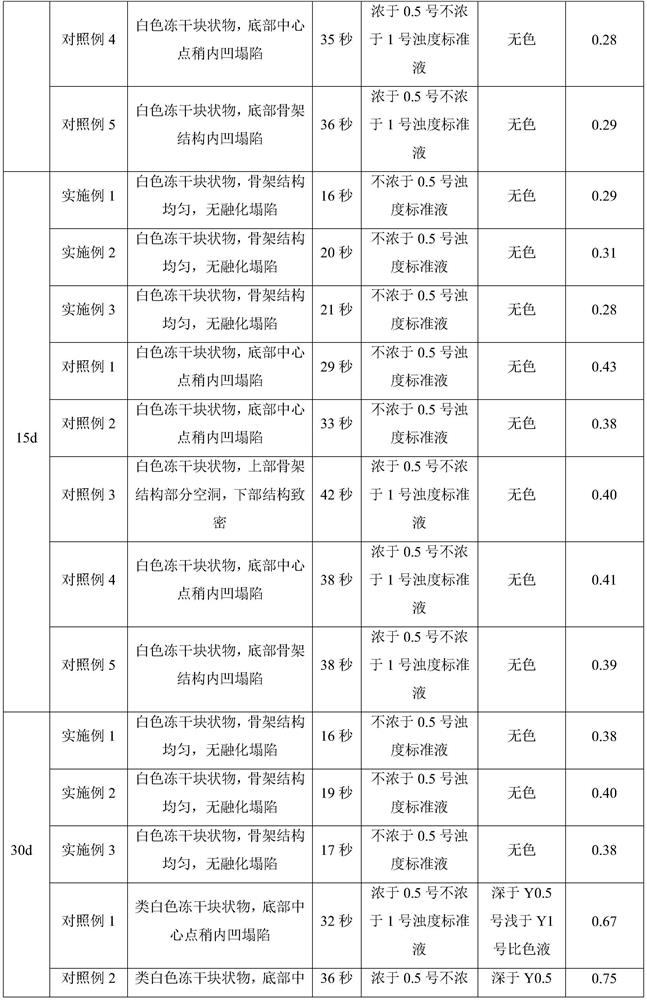
Image
Examples
Embodiment 1
[0028] (1) Prepare 80% water for injection in the container; add 40.3g sodium hydroxide and stir to dissolve; add 250g (166.7mg / ml) ganciclovir and stir to dissolve; add 10% hydrochloric acid solution to adjust the pH value to 11.2, and then Add water for injection to 1.5 L, stir evenly to obtain Ganciclovir liquid medicine, and liquid medicine obtains Ganciclovir sample after aseptic filtration and canning (1.5mL / bottle).
[0029] (2) The ganciclovir samples were pre-frozen. During pre-freezing, the plate layer was rapidly cooled from room temperature to -45°C and kept for 1.5 hours, with a cooling rate of 75°C / h. The pre-frozen ganciclovir samples were subjected to sublimation drying. During sublimation drying, the plate layer was heated from -45°C to -30°C and kept for 9 hours. environment. The ganciclovir after sublimation drying is analyzed and dried. During the analysis and drying, the temperature of the plate layer is raised from -30°C to 50°C and kept for 4 hours. in...
Embodiment 2
[0031] (1) Prepare 80% water for injection in the container; add 37.1g sodium hydroxide and stir to dissolve; add 230g (153.3mg / ml) ganciclovir and stir to dissolve; add 10% hydrochloric acid solution to adjust the pH value to 11.3, then Add water for injection to 1.5 L, stir evenly to obtain Ganciclovir liquid medicine, and liquid medicine obtains Ganciclovir sample after aseptic filtration and canning (1.5mL / bottle).
[0032] (2) The ganciclovir samples were pre-frozen. During pre-freezing, the plate layer was rapidly cooled from room temperature to -45°C and kept for 1 hour, with a cooling rate of 70°C / h. The pre-frozen ganciclovir samples were subjected to sublimation drying. During sublimation drying, the plate layer was heated from -45°C to -30°C and kept for 10 hours. environment. The ganciclovir after sublimation drying is analyzed and dried. During the analysis and drying, the temperature of the plate layer is raised from -30°C to 50°C and kept for 3 hours. in progr...
Embodiment 3
[0034] (1) Prepare 80% water for injection in the container; add 41.9g sodium hydroxide and stir to dissolve; add 260g (173.3mg / ml) ganciclovir and stir to dissolve; add 10% hydrochloric acid solution to adjust the pH value to 11.5, then Add water for injection to 1.5 L, stir evenly to obtain Ganciclovir liquid medicine, and liquid medicine obtains Ganciclovir sample after aseptic filtration and canning (1.5mL / bottle).
[0035] (2) The ganciclovir samples were pre-frozen. During pre-freezing, the plate layer was rapidly cooled from room temperature to -45°C and kept for 2 hours, with a cooling rate of 80°C / h. The pre-frozen ganciclovir samples were subjected to sublimation drying. During sublimation drying, the plate layer was heated from -45°C to -30°C and kept for 8 hours. The heating rate was 5°C / h. environment. The ganciclovir after sublimation drying is analyzed and dried. During the analysis and drying, the temperature of the plate layer is raised from -30°C to 50°C and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com