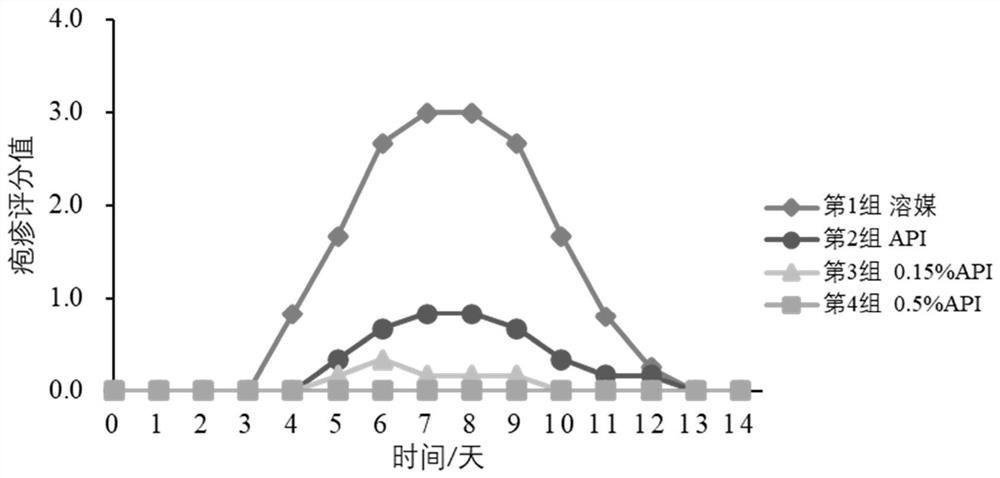
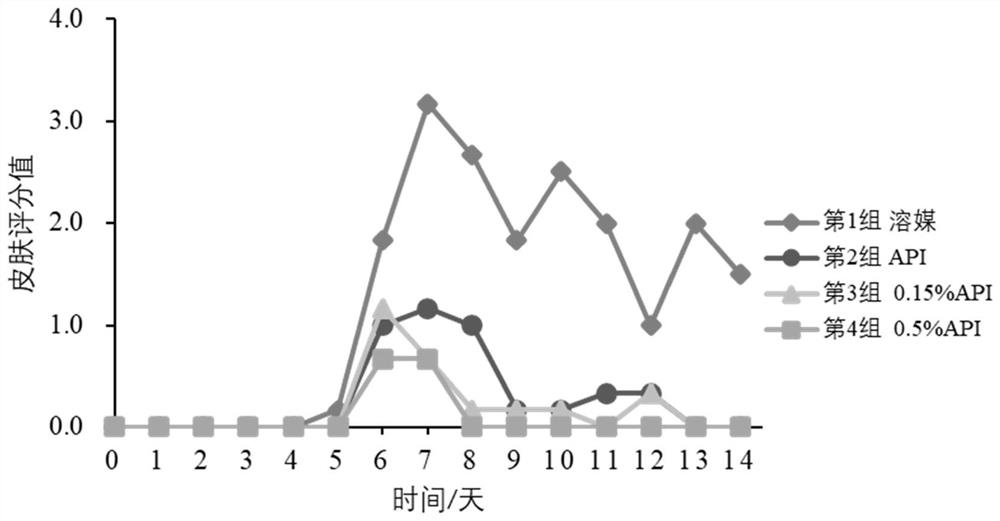
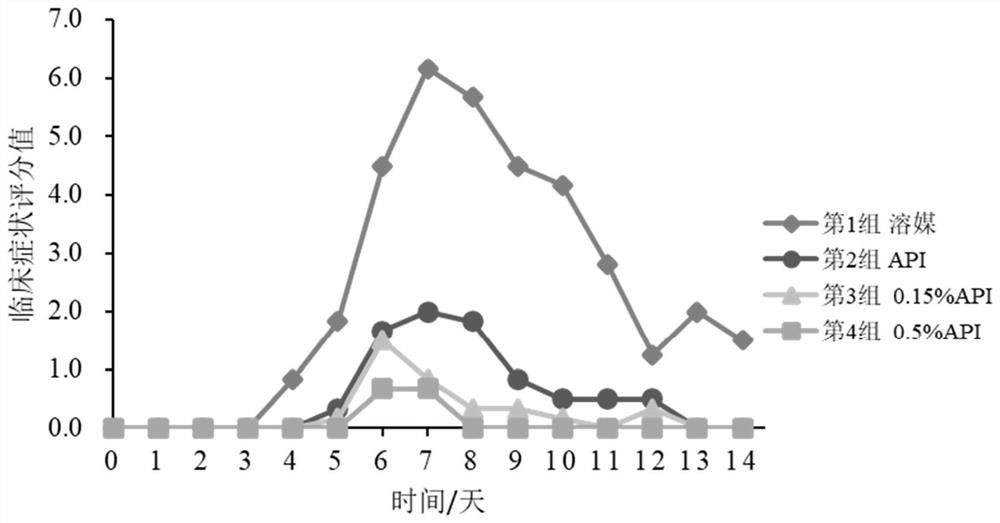
External preparation of ganciclovir and application thereof
An external preparation, the technology of ganciclovir, applied in the field of medicine, can solve the problems of irregular recurrence, inability to be completely cured, HSV-2 can not be cured, etc., and achieve the effect of excellent antiviral effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0032] The selection of embodiment 1.1 auxiliary material kind
[0033] In the research and development process, the inventor has carried out the compatibility test of raw materials and auxiliary materials, wherein Table 1 is the ratio of raw materials and auxiliary materials, and Table 2 is the stability test.
[0034] Table 1 Proportion of raw and auxiliary materials
[0035]
[0036]
[0037] Prepare according to the ratio of raw materials and auxiliary materials in Table 1 respectively, mix each raw material and auxiliary materials evenly, melt the solid oil phase matrix and mix it with API (i.e. ganciclovir), directly mix the liquid matrix, and mix the water phase solid auxiliary material preparation with API mix. The mixed materials were placed under light and high temperature conditions of 40°C, and samples were taken for testing after 10 days. The results are shown in Table 2.
[0038] Table 2 Stability test results
[0039]
[0040]
[0041] The result...
Embodiment 12
[0042] Example 1.2 The selection of the amount of auxiliary materials—the screening of the amount of polymer matrix (carbomer)
[0043] In this example, taking carbomer as an example, the influence of dosage on the gel preparation was studied, and the prescriptions in Table 3 were screened.
[0044] table 3
[0045]
[0046] Preparation method: (1) Add the prescribed amount of carbomer into purified water accounting for 40% of the total weight, stir to fully swell, and form a uniform dispersion;
[0047] (2) After dissolving sodium hydroxide, ganciclovir, benzalkonium chloride and glycerin with purified water accounting for 50% of the total weight, add the filtrate to the dispersion in step (1), and stir while adding;
[0048] (3) Use triethanolamine to adjust the pH value to about 7, add purified water to a sufficient amount (100%), and stir to form a uniform gel.
[0049] Observe or measure the character, pH value, and viscosity of the product prepared above. The resul...
Embodiment 1
[0053] The determination of the pH value range of embodiment 1.3 gel
[0054] The external preparation of the present invention belongs to a semi-solid preparation, and the pH value is a key quality attribute. Therefore, the pH value range of the external preparation of the present invention is screened to determine a suitable pH range that can make the preparation stable. The pH value of human skin is about It is 5.5~7.0, and ganciclovir is unstable under acidic conditions, so the proposed investigation range of the present invention is 5.5~7.5. The recipes used are shown in Table 5.
[0055] table 5
[0056]
[0057] Preparation method: (1) Add the prescribed amount of carbomer into purified water accounting for 40% of the total weight, stir to fully swell, and form a uniform dispersion;
[0058] (2) After dissolving sodium hydroxide, ganciclovir, benzalkonium chloride and glycerin with purified water accounting for 50% of the total weight, add the filtrate to the dispe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com