Method for preparing and purifying Valganciclovir hydrochloride
A technology of valganciclovir hydrochloride and weight, applied in the field of valganciclovir hydrochloride, can solve the problems of troublesome post-processing, increased cost, and large potential safety hazard, and achieves easy and convenient post-processing operation, reduced reaction steps, and potential safety hazard. big effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
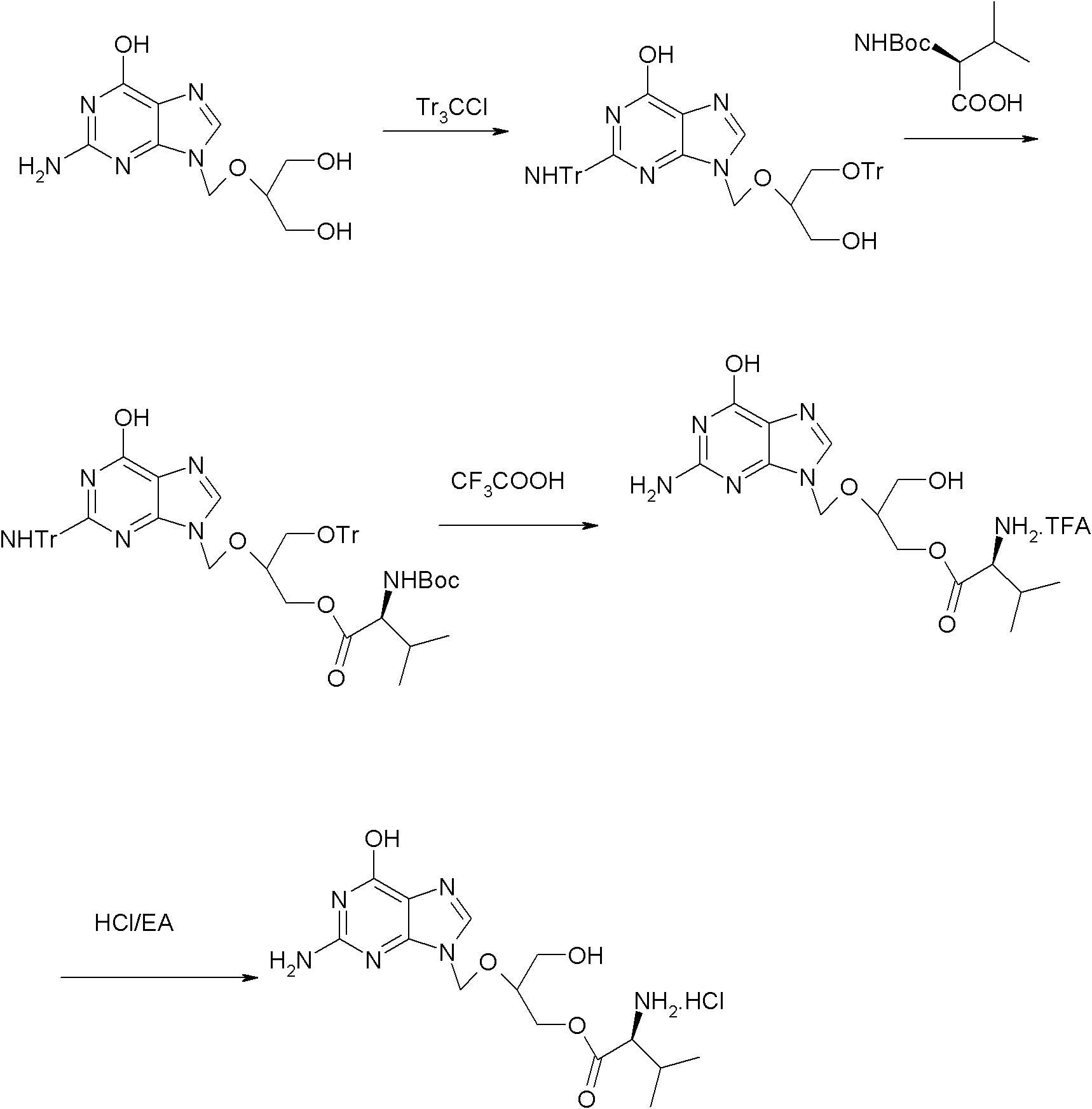
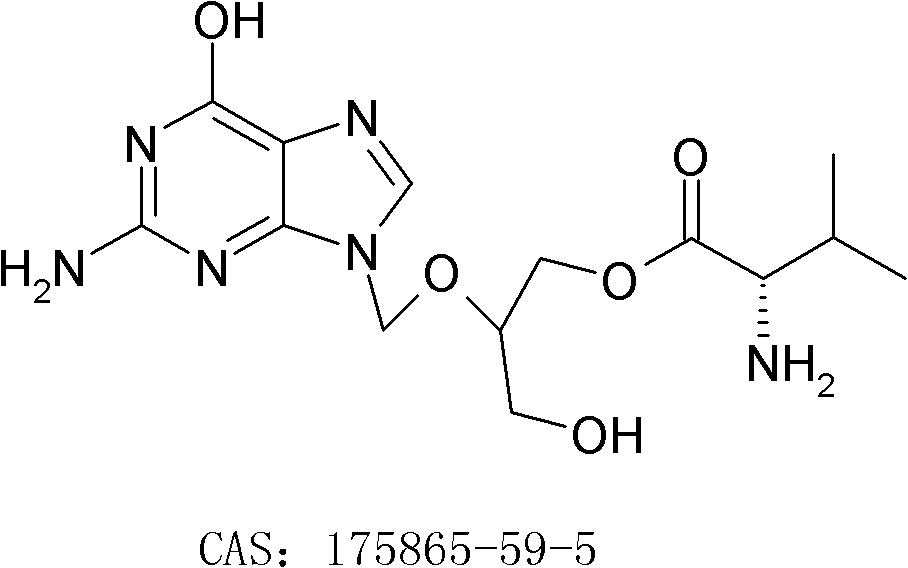
[0026] Embodiment 1: the preparation of valganciclovir hydrochloride
[0027] Step 1: Preparation of N,O-trityl ganciclovir
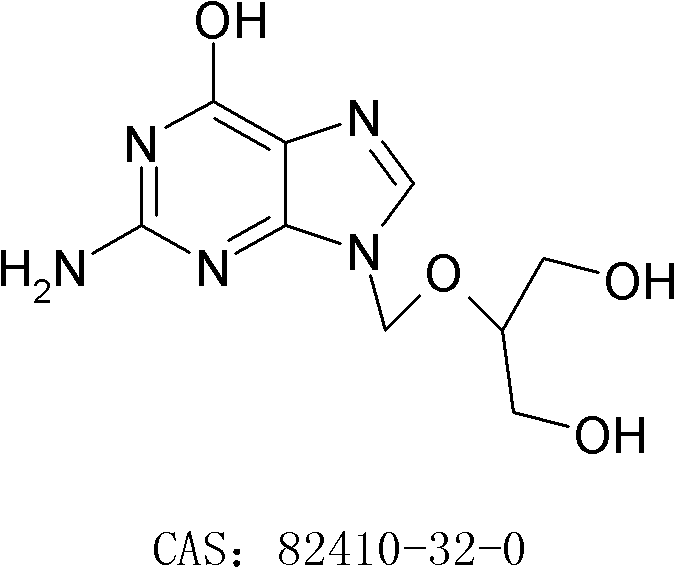
[0028] Add 82g of ganciclovir, 770g of DMF, 173g of TEA and 0.8g of DMAP in the reaction flask, stir evenly to form a white suspension, heat up to about 50°C, and add dropwise 238g of trityl chloride and 770g DMF solution, after adding, keep warm at 45-50°C and stir for 15 hours; after the reaction is completed, cool down to 10°C and stir for 30 minutes, filter, rinse the solid with 150g DMF, combine the filtrate, slowly add 1750g water, finish adding, and leave at room temperature After stirring for 2 hours, it was filtered, and the obtained solid was stirred and washed with 350 g of water and 350 g of ethyl acetate in turn, and dried to obtain 146.8 g of off-white solid, yield: 61.7%.
[0029] Step 2: Preparation of N, O-trityl-O-(N-Boc-L-valine) ganciclovir
[0030] Add 28.21g of N-Boc-L-valine, 100g of DMF, and 14g of TEA into the reaction flask, ...
Embodiment 2
[0036] Embodiment 2: the preparation of valganciclovir hydrochloride
[0037] Step 1: Preparation of N,O-trityl ganciclovir
[0038] Add 200g of ganciclovir, 1600g of DMF, 500g of TEA and 1.0g of DMAP in the reaction flask, stir evenly to form a white suspension, heat up to about 55°C, and add dropwise 540g of trityl chloride and 1600g of DMF solution, after adding, keep warm at 48-55°C and stir for 12 hours; after the reaction, cool down to 10°C and stir for 30 minutes, filter, rinse the solid with 400g DMF, combine the filtrate, slowly add 4500g of water, finish adding, in After stirring at room temperature for 2 hours, it was filtered, and the obtained solid was washed with 800 g of water and 800 g of ethyl acetate in turn, and dried to obtain 368.9 g of off-white solid, yield: 63.6%.
[0039] Step 2: Preparation of N, O-trityl-O-(N-Boc-L-valine) ganciclovir
[0040] Add 108g of N-Boc-L-valine, 432g of DMF, and 72g of TEA into the reaction flask, stir evenly, cool down to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com