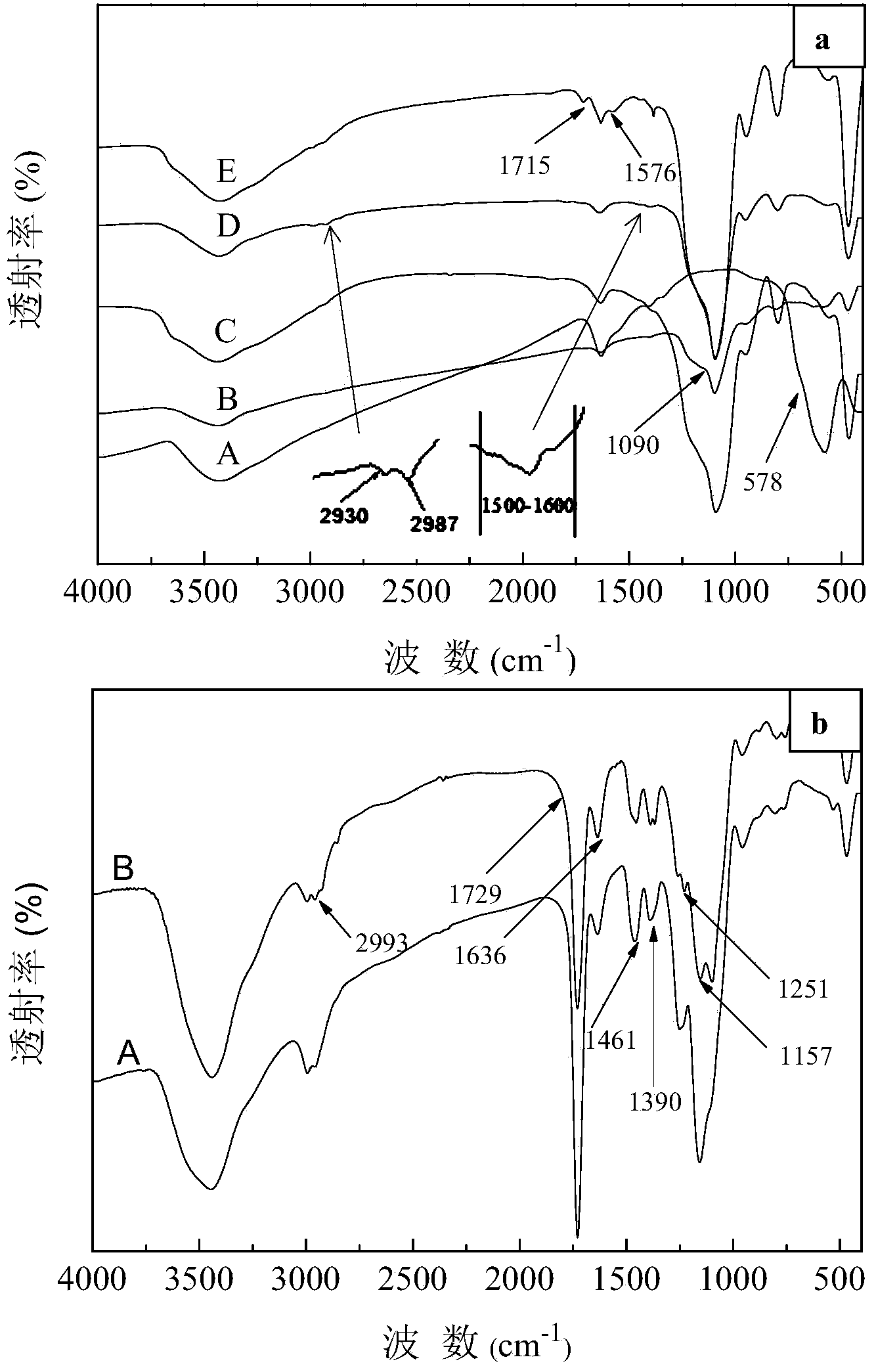
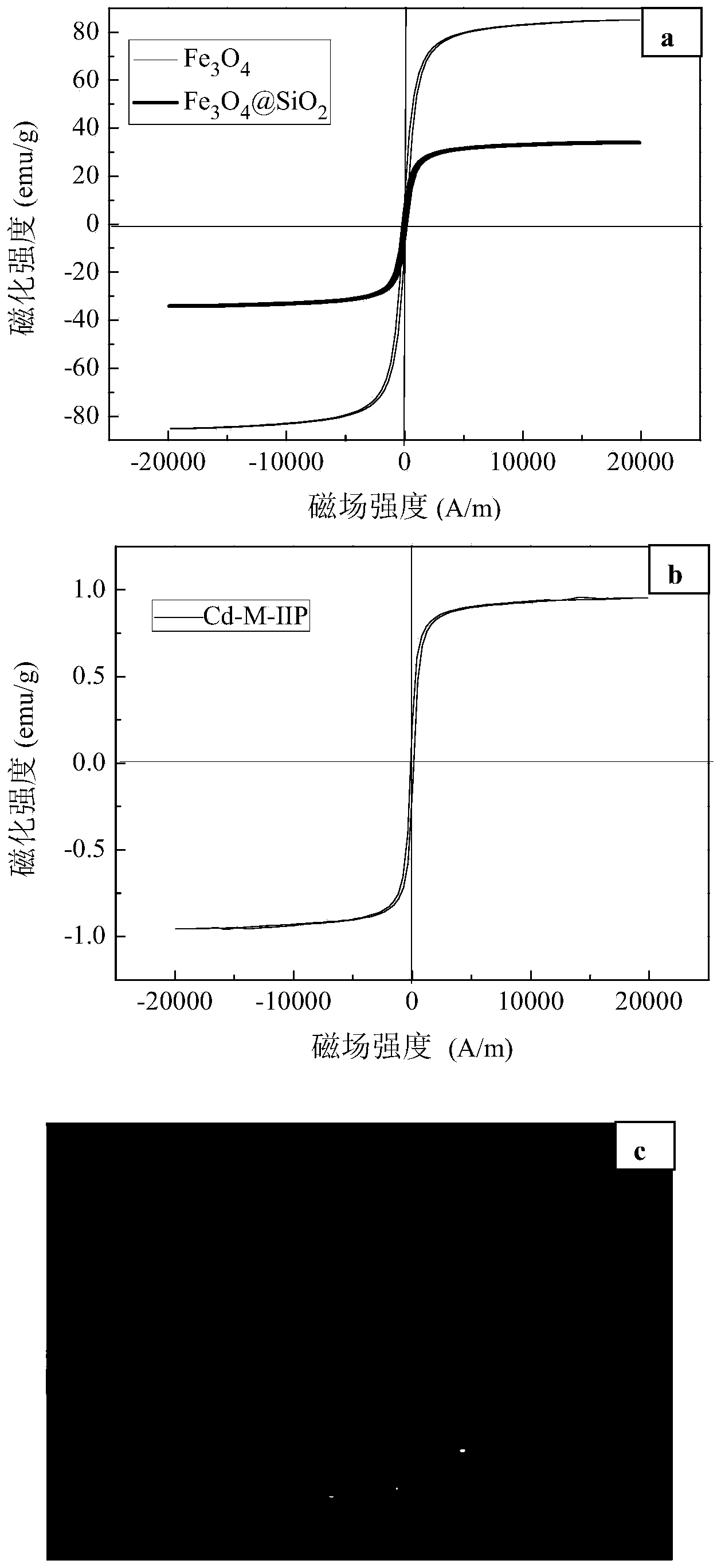
Preparation method of magnetic cadmium ion surface imprinted polymer
A surface imprinting, cadmium ion technology, applied in the directions of alkali metal compounds, chemical instruments and methods, alkali metal oxides/hydroxides, etc., can solve the problems of poor rigidity, thermal instability, limited hydroxyl content, etc. The effect of improving adsorption capacity, improving introduction efficiency, and improving adsorption performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Fe 3 o 4 Synthesis of: 2.78g FeSO 4 ·7H 2 O and 4.73gFeCl 3 ·6H 2 O dissolved in 100g ultrapure water (ultrapure water is water that almost completely removes the conductive medium in water, and removes non-dissociated colloidal substances, gases and organic substances in water to a very low level. The resistivity is greater than 18MΩ*cm, Or close to the limit value of 18.3MΩ*cm.) In, through N 2 Under the condition of vigorous stirring (stepless speed regulation booster agitator, the speed regulation gear is set to 2.5 (DW-Ⅱ type, Yuhua Instrument Co., Ltd., Gongyi City), add 10g of 25-28% mass percent Ammonia water, react at 60°C for 1 hour, add 0.5g citric acid to react for 60 minutes, magnetically separate after cooling, collect the black sediment, remove the supernatant, wash with ultrapure water until neutral, then wash with 50mL absolute ethanol for 2 to 3 times, Vacuum dried to obtain Fe 3 o 4 Nanoparticles; (2) Fe 3 o 4 SiO 2 Synthesis of microsp...
Embodiment 2
[0035] (1) Fe 3 o 4 Synthesis of: 5.56g FeSO 4 ·7H 2 O and 9.46 g FeCl 3 ·6H 2 O dissolved in 200g ultrapure water, pass N 2 Under the conditions, add 20g of ammonia water with a mass percentage concentration of 25-28% under vigorous stirring, react at 60°C for 1h, add 1g of citric acid for 90min, and magnetically separate after cooling, collect the black sediment, remove the supernatant, and use ultra-pure Wash with water until neutral, then wash with 50mL absolute ethanol for 2 to 3 times, and dry in vacuum to obtain Fe 3 o 4 Nanoparticles; (2) Fe 3 o 4 SiO 2 Synthesis of microspheres: 2gFe 3 o 4 Ultrasonic dispersion of nanoparticles in a mixed solution consisting of 158g methanol and 40g distilled water, add 8g ammonia water and 14g ethyl orthosilicate, react at room temperature for 24h, magnetically separate the product, collect the sediment, remove the upper solution, successively wash with 50mL distilled water and 50mL Wash with absolute ethanol 2 to 3 times...
Embodiment 3
[0037] (1) Fe 3 o 4 Synthesis of: 2.78g FeSO 4 ·7H 2 O and 4.73gFeCl 3·6H 2 Dissolve O in 100g ultrapure water, pass N 2 Under the conditions, add 15g of ammonia water with a mass percentage concentration of 25-28% under vigorous stirring, react at 70°C for 1h, add 0.5g of citric acid for 60min, and magnetically separate after cooling, collect the black sediment, remove the supernatant, and use supernatant Wash with pure water until neutral, then wash with 50mL absolute ethanol for 2 to 3 times, and dry in vacuum to obtain Fe 3 o 4 Nanoparticles; (2) Fe 3 o 4 SiO 2 Synthesis of microspheres: 1 gFe 3 o 4 Ultrasonic dispersion of nanoparticles in a mixed solution consisting of 79g of absolute ethanol and 20g of distilled water, add 3g of ammonia water and 8g of ethyl orthosilicate, react at room temperature for 12h, magnetically separate the product, collect the sediment, remove the upper solution, and successively wash with 50mL of distilled water and 50 mL of absol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com