Fe2O3 micro-nano porous sphere, preparation method thereof and uses thereof
A ferric oxide, micro-nano technology, applied in iron oxide, chemical instruments and methods, iron oxide/iron hydroxide and other directions, can solve the problems of restricting popularization and application, difficult practical application, low energy utilization rate, etc. Simple, short time, convenient post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The concrete steps of preparation are:
[0034] Step 1: first mix ferric chloride hexahydrate, ascorbic acid, urea and water according to the molar ratio of 0.8:1.2:2:700 and then stir evenly; wherein, the water is deionized water to obtain a mixed solution. Then the mixed solution was reacted for 6 hours at a temperature of 140° C. and an autogenous pressure to obtain an intermediate product.
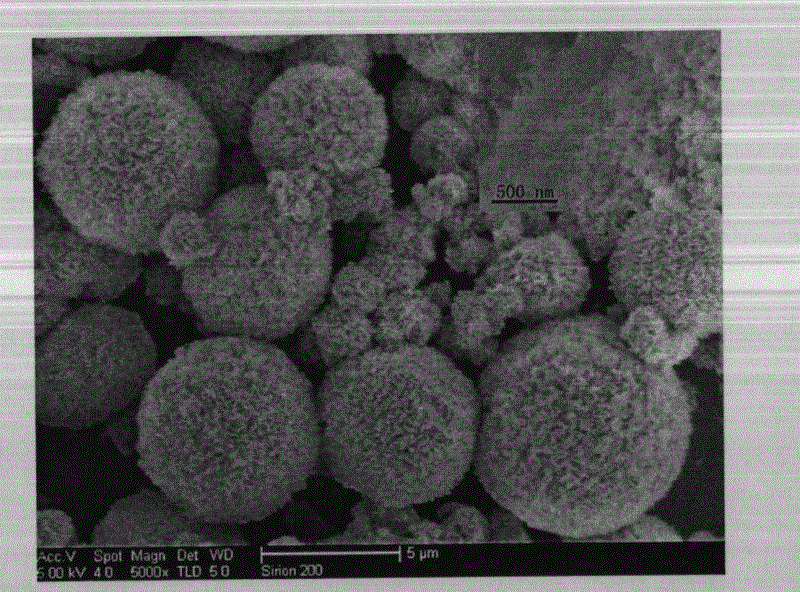
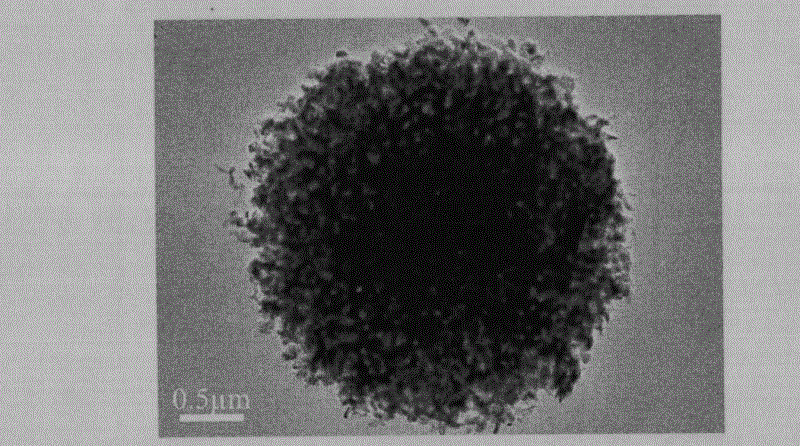
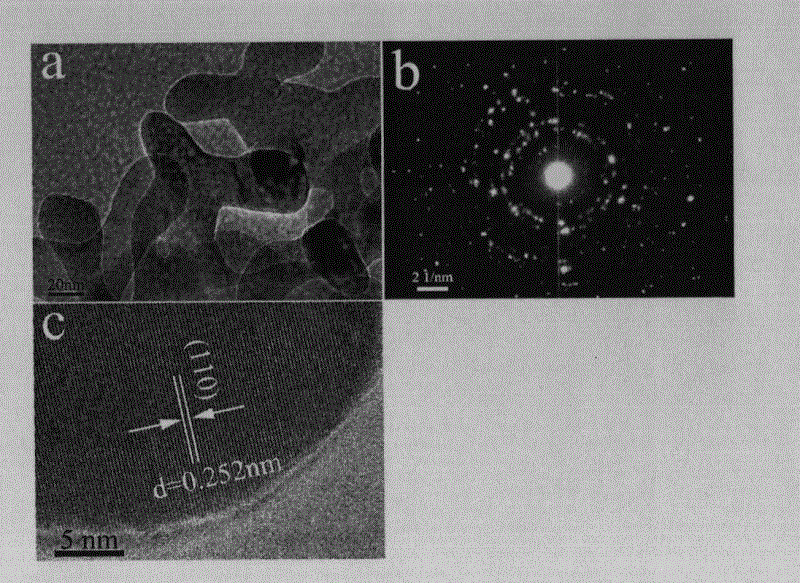
[0035] Step 2, the intermediate product is first separated, washed and dried; wherein, the separation process is centrifugation (or magnetic separation), the speed of centrifugation is 3500r / min, the time is 3min, and the washing process is to use deionized water Wash and separate the obtained powder until it becomes neutral, and dry at 75° C. for 2 hours to obtain porous iron carbonate. Then the porous iron carbonate was annealed at 450°C for 6h and then naturally cooled to room temperature; the time for heating to 450°C was 30min, and the obtained figure 1 , figure 2 and ...
Embodiment 2
[0037] The concrete steps of preparation are:
[0038] Step 1: first mix ferric chloride hexahydrate, ascorbic acid, urea and water according to the molar ratio of 0.9:1.1:2.3:650 and then stir evenly; wherein, the water is distilled water to obtain a mixed solution. Then, the mixed solution was reacted for 5.5 hours at a temperature of 150° C. and an autogenous pressure to obtain an intermediate product.
[0039] Step 2, the intermediate product is first separated, washed and dried; wherein, the separation process is centrifugation (or magnetic separation), the speed of centrifugation is 5000r / min, the time is 2.5min, and the washing process is to use distilled water to clean The powder obtained after the separation treatment is neutral, and the drying treatment is to dry at 78° C. for 2 hours to obtain porous iron carbonate. Then the porous iron carbonate was annealed at 480°C for 5.5h and then cooled to room temperature naturally; the time for heating to 480°C was 30min, a...
Embodiment 3
[0041] The concrete steps of preparation are:
[0042] Step 1: first mix ferric chloride hexahydrate, ascorbic acid, urea and water according to a molar ratio of 1:1:2.5:600 and then stir evenly; wherein, the water is deionized water to obtain a mixed solution. Then the mixed solution was reacted for 5 hours at a temperature of 160° C. and an autogenous pressure to obtain an intermediate product.
[0043] Step 2, the intermediate product is first separated, washed and dried; wherein, the separation process is centrifugation (or magnetic separation), the speed of centrifugation is 6000r / min, the time is 2min, and the washing process is to use deionized water Wash and separate the obtained powder until it becomes neutral, and dry at 80° C. for 2 hours to obtain porous iron carbonate. Then, the porous iron carbonate was annealed at 500°C for 5h and then cooled to room temperature naturally; the time for heating up to 500°C was 30min, and the following figure 1 , figure 2 and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com