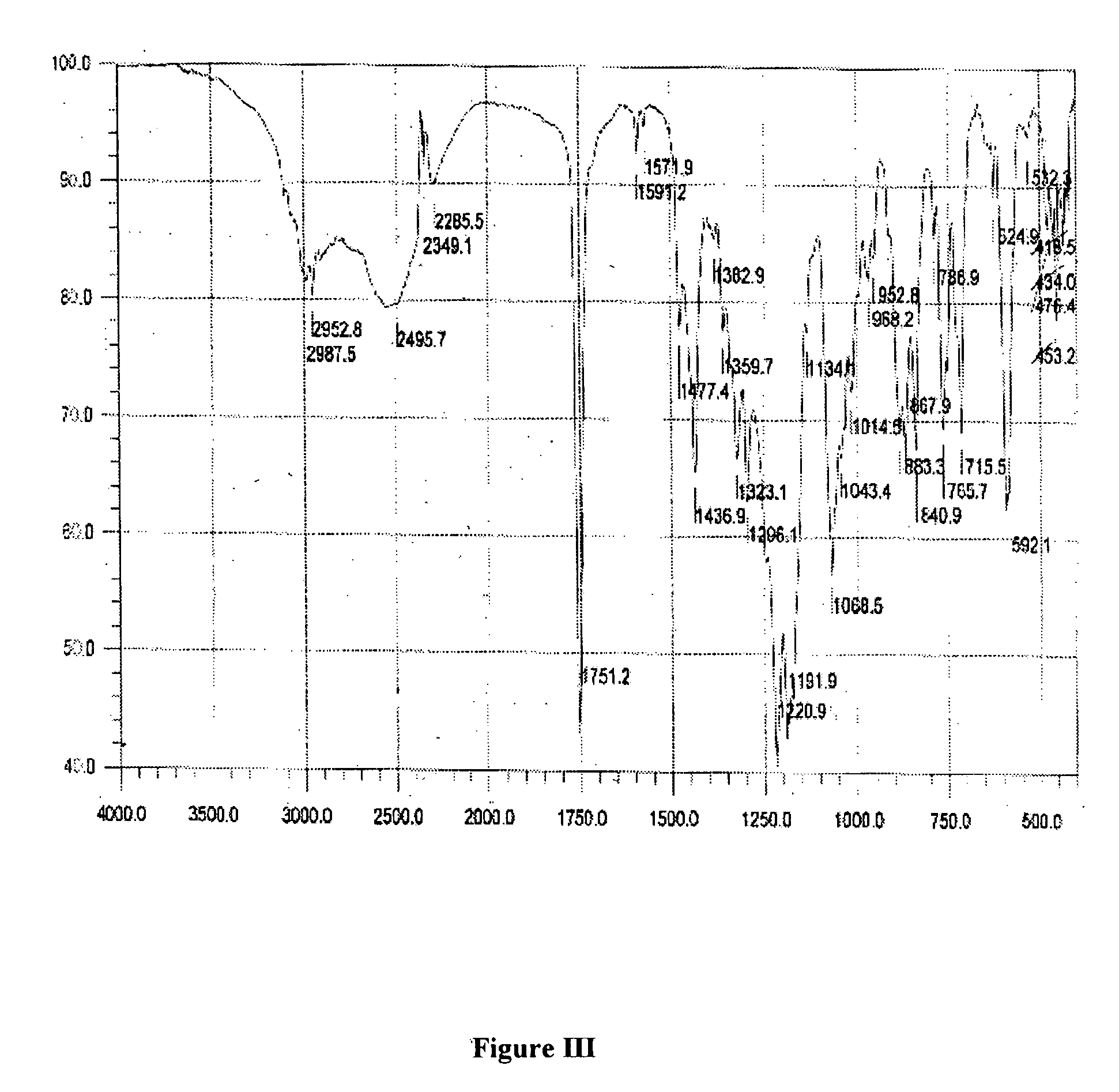
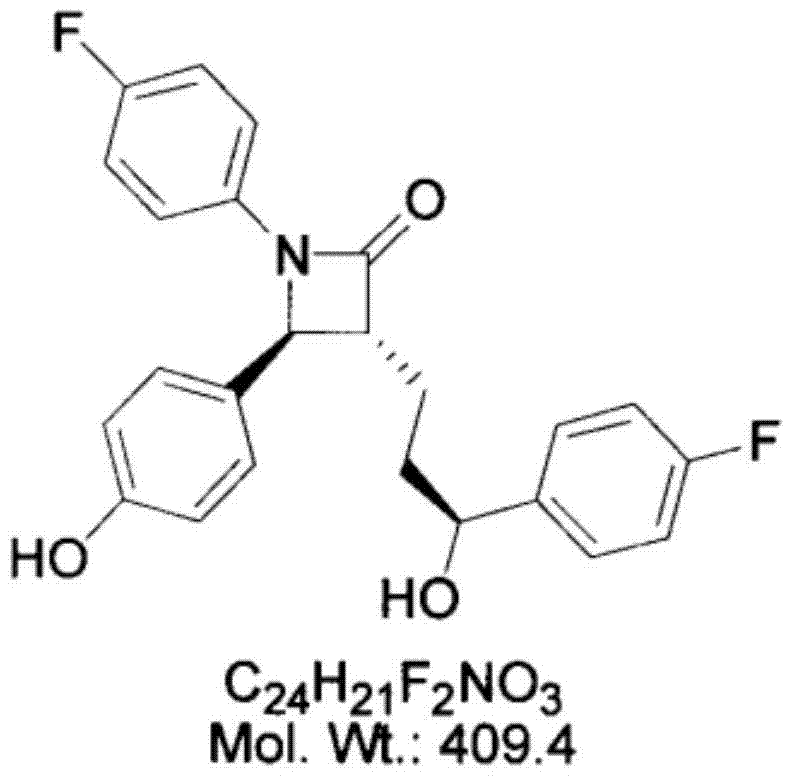
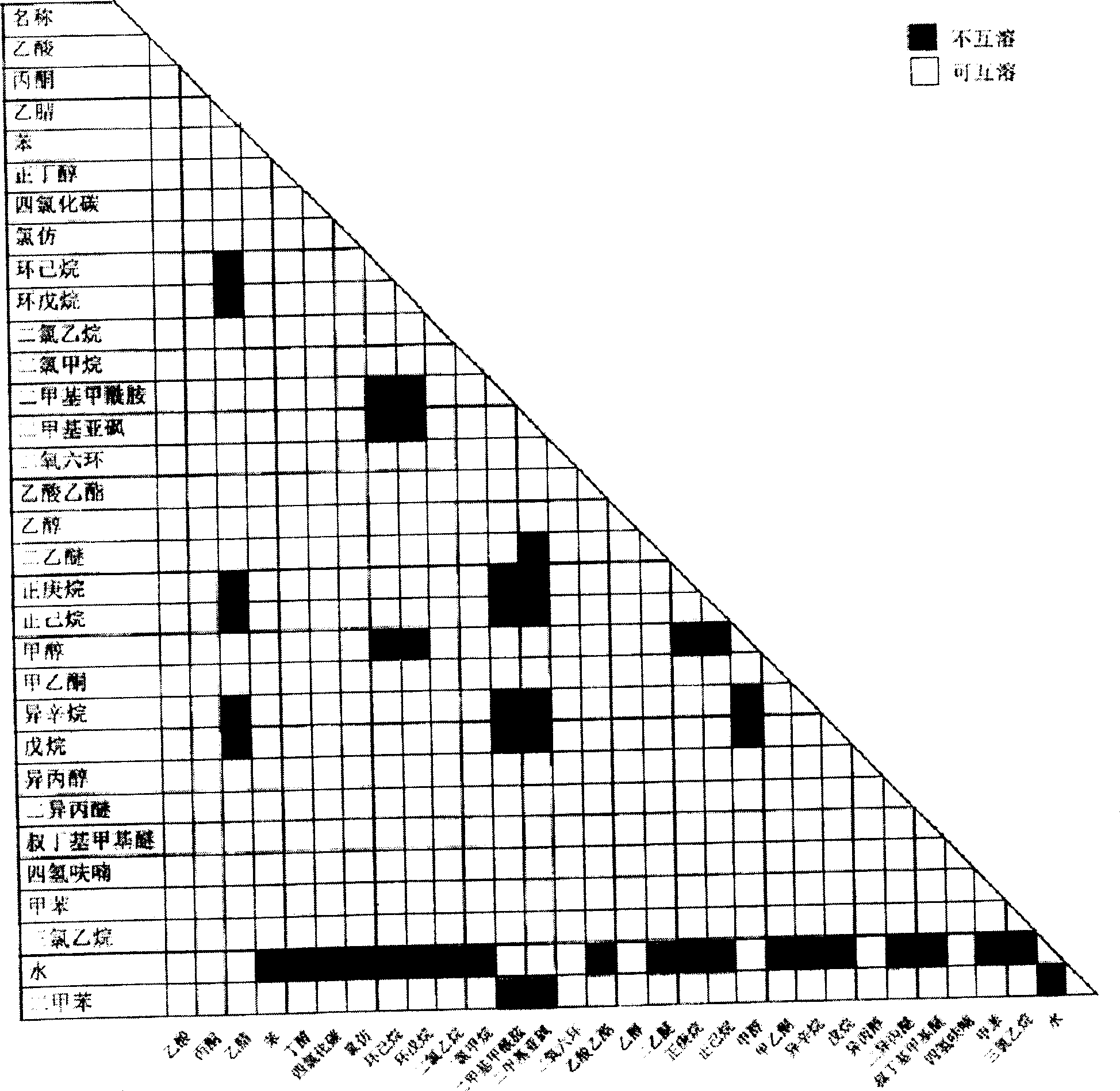
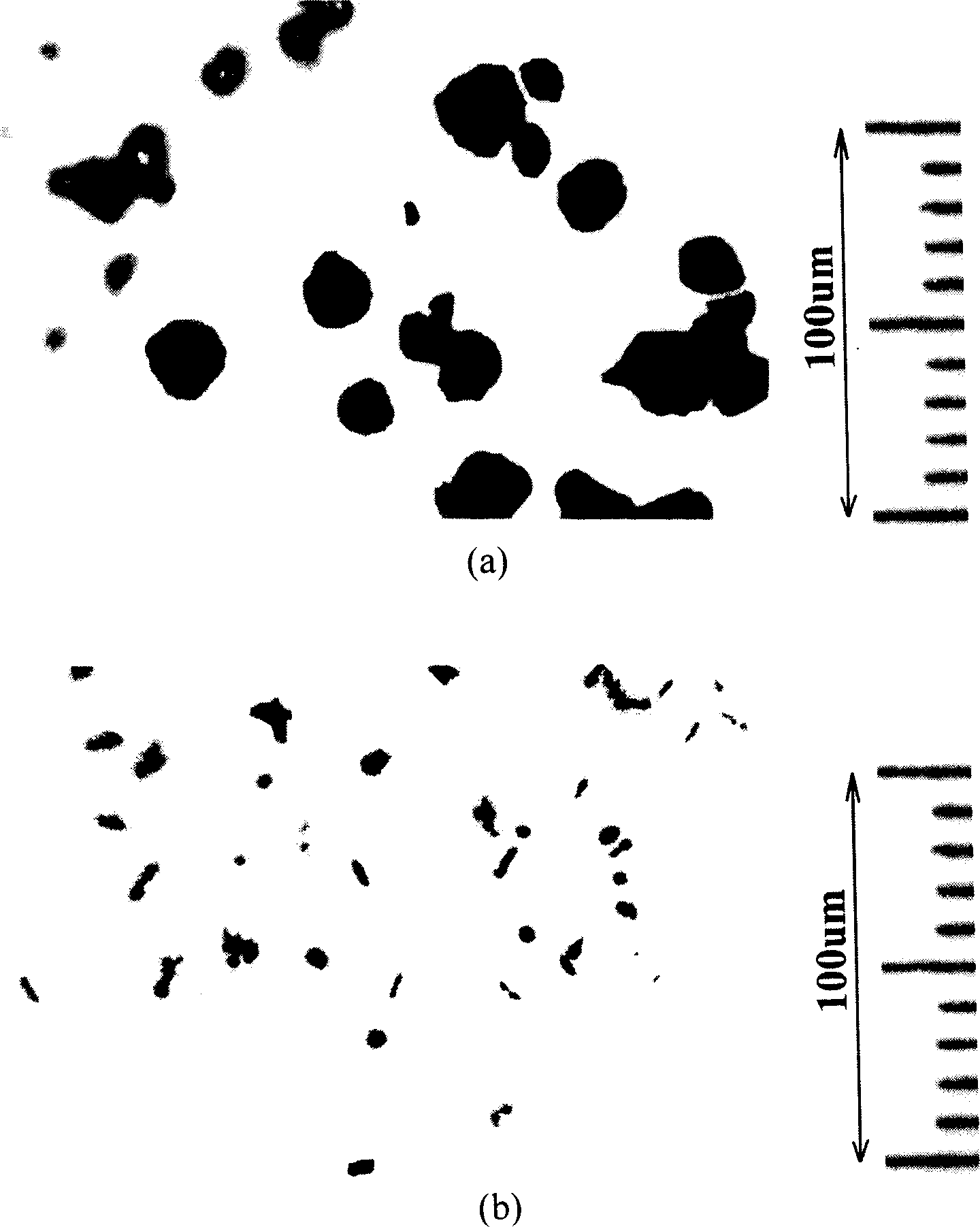
Patents
Literature
597 results about "Anti solvent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lyophilization process and products obtained thereby
ActiveUS20070116729A1Dissolve fastSuitable for useBiocidePowder deliveryHigh concentrationFreeze-drying
A lyophilization process which comprises dissolving a material in one or more solvents for said material to form a solution; forcing said material at least partially out of solution by combining the solution and a non-solvent for the material, which non-solvent is miscible with the solvent or solvents used and wherein said non-solvent is volatilizable under freeze-drying conditions. In addition, for hydrophobic and / or lipophilic materials, the anti-solvent can be omitted, and the solution of the material in the solvent can be subjected directly to freeze drying. The lyophilizates can then be reconstituted with typical aqueous diluent in the case of hydrophilic materials. Hydrophobic and or lipophilic materials can be initially reconstituted with propylene glycol and / or polyethyleneglycol to form a high concentration solution therein and this is further diluted for use with a diluent of Intralipid, plasma, serum, or even whole blood.
Owner:SCIDOSE PHARMA +1
Method of preparing a poly(arylene ether), and a poly(arylene ether) prepared thereby
A method of preparing a poly(arylene ether) includes oxidatively polymerizing a monohydric phenol in solution, concentrating the solution by removing a portion of the solvent to form a concentrated solution having a cloud point, Tcloud, and combining the concentrated solution with an anti-solvent to precipitate the poly (arylene ether), wherein the concentrated solution has a temperature of at least about (Tcloud-10° C.) immediately before it is combined with the anti-solvent. The method reduces the formation of undesirably fine particles in the product poly(arylene ether).
Owner:SHPP GLOBAL TECH BV
Method and apparatus for treating a substrate with dense fluid and plasma
ActiveUS20060278254A1High densityDivergent cohesion energyElectric discharge tubesElectric arc lampsSolid phasesWet cleaning
The present invention is a method, process and apparatus for selective cleaning, drying, and modifying substrate surfaces and depositing thin films thereon using a dense phase gas solvent and admixtures within a first created supercritical fluid anti-solvent. Dense fluids are used in combination with sub-atmospheric, atmospheric and super-atmospheric plasma adjuncts (cold and thermal plasmas) to enhance substrate surface cleaning, modification, precision drying and deposition processes herein. Moreover, conventional wet cleaning agents such as hydrofluoric acid and ammonium fluoride may be used with the present invention to perform substrate pre-treatments prior to precision drying and cleaning treatments described herein. Finally, dense fluid such as solid phase carbon dioxide and argon may be used as a follow-on treatment or in combination with plasmas to further treat a substrate surface.
Owner:HITACHI HIGH-TECH CORP
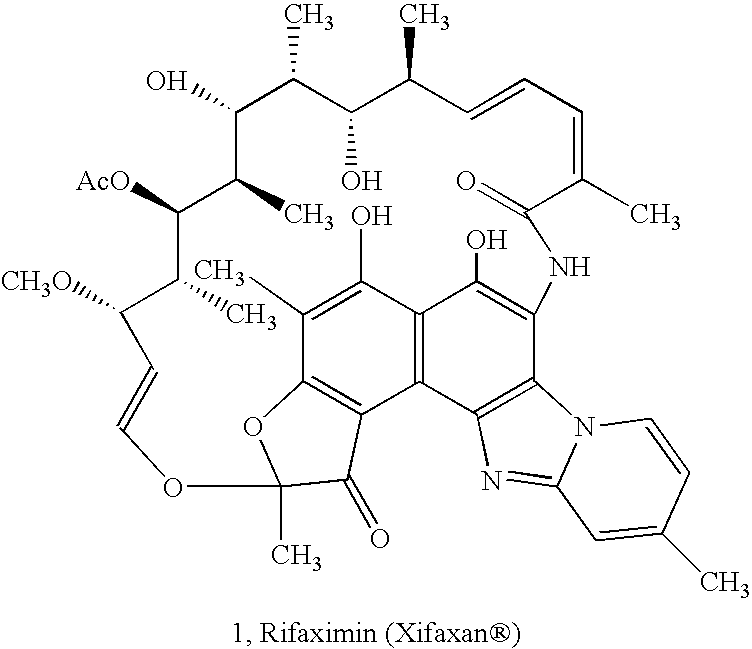
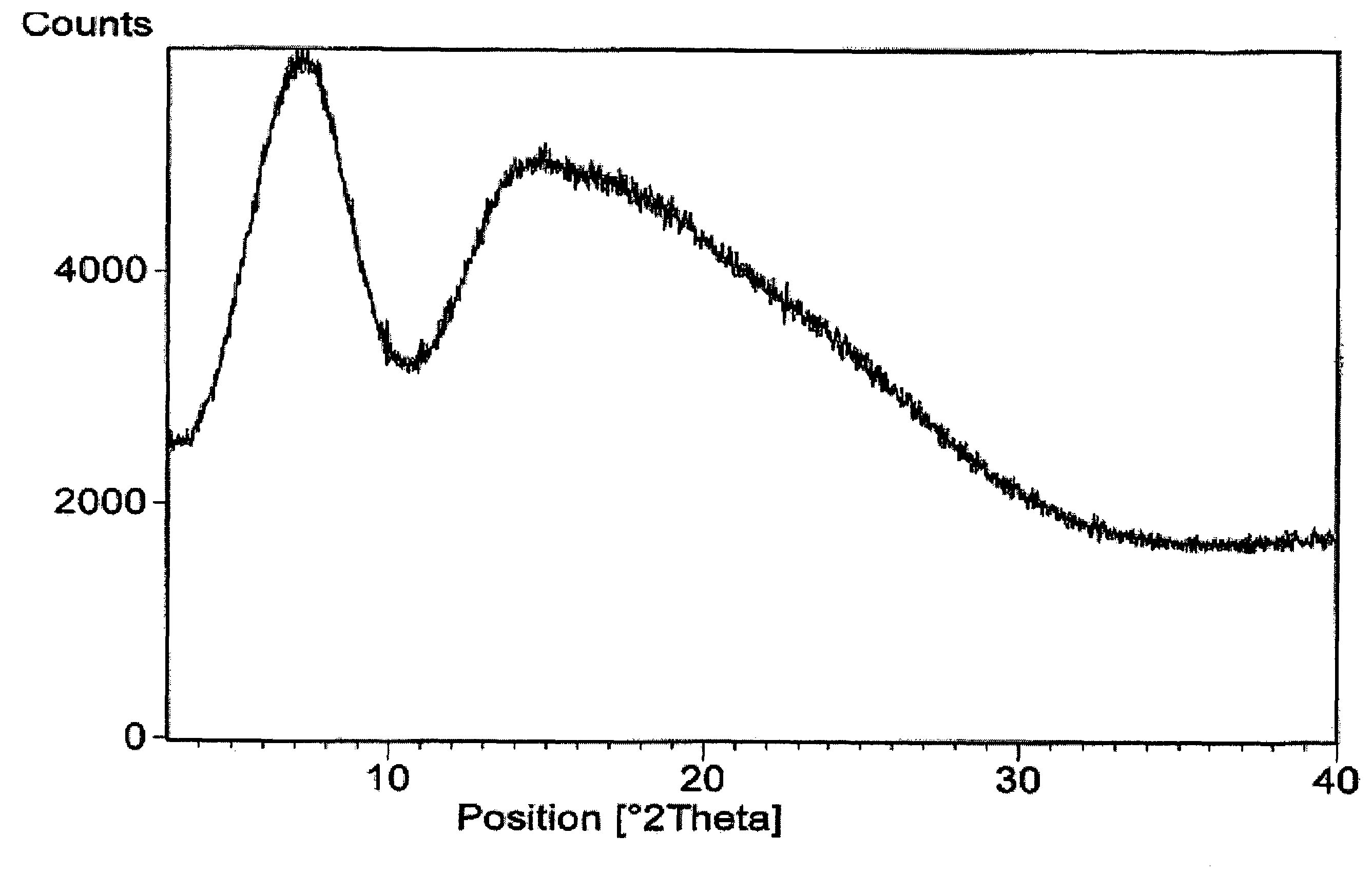
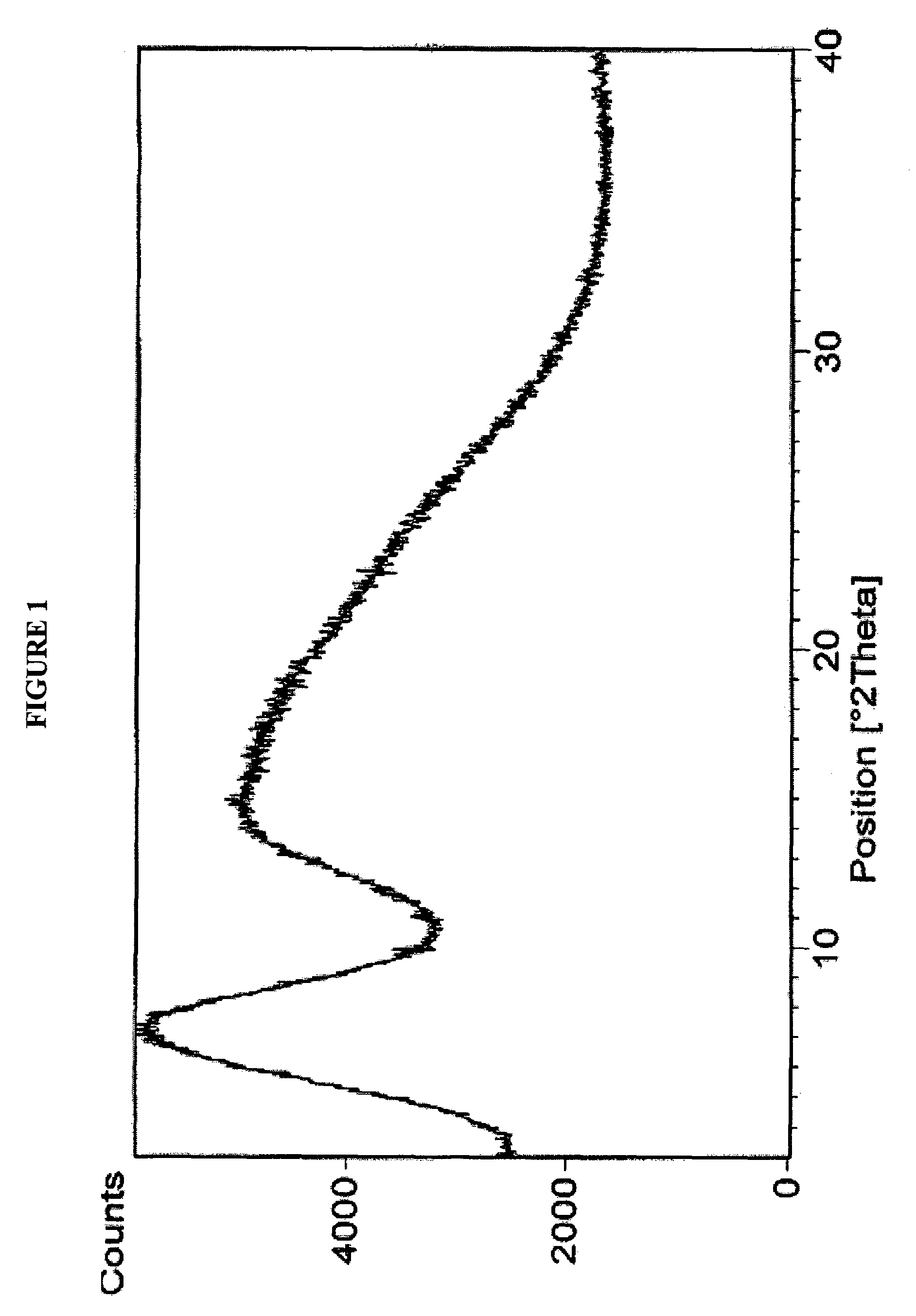
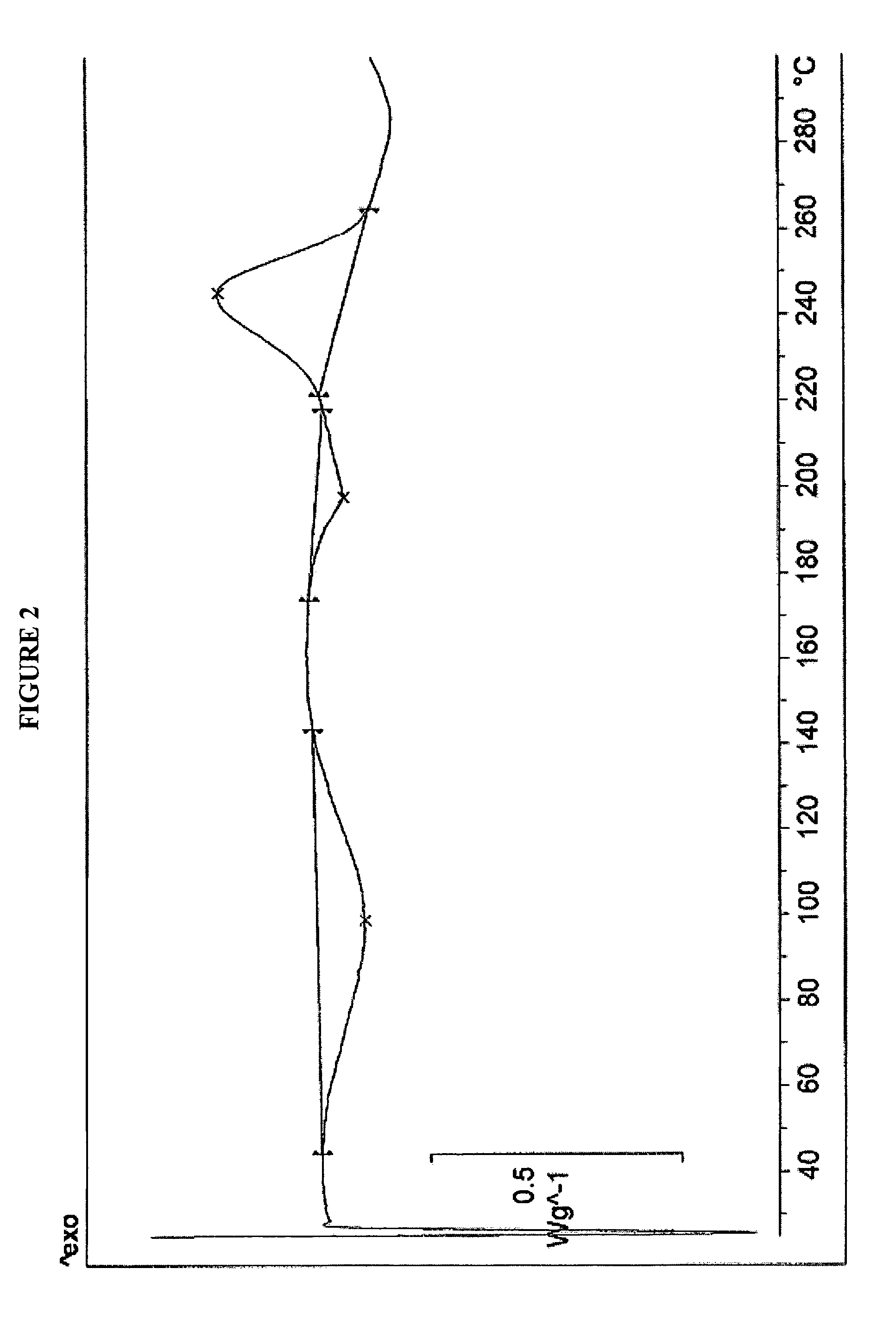
Amorphous form of rifaximin and processes for its preparation
A stable amorphous form of rifaximin is disclosed. This form is chemically and polymorphically stable on storage and can be prepared by dissolving rifaximin in a solvent to form a solution, which is precipitated by adding an anti-solvent and isolating of the precipitated amorphous rifaximin as an end product.
Owner:SALIX PHARMA INC
Amorphous form of rifaximin and processes for its preparation
A stable amorphous form of rifaximin is disclosed. This form is chemically and polymorphically stable on storage and can be prepared by dissolving rifaximin in a solvent to form a solution, which is precipitated by adding an anti-solvent and isolating of the precipitated amorphous rifaximin as an end product.
Owner:SALIX PHARMA INC
Particle formation
InactiveUS20030109421A1Risk minimizationPeptide/protein ingredientsGranulation by liquid drop formationAnti solventEngineering
Method for preparing a target substance in particulate form, comprising introducing into a particle formation vessel, through separate first and second fluid inlets respectively, (a) a "target solution / suspension" of the substance in a fluid vehicle and (b) a compressed fluid anti-solvent, and allowing the anti-solvent to extract the vehicle so as to form particles of the substance, wherein the anti-solvent fluid has a sonic, near-sonic or supersonic velocity as it enters the vessel, and wherein the anti-solvent and the target solution / suspension enter the vessel at different locations and meet downstream (in the direction of anti-solvent flow) of the second fluid inlet. Also provided is apparatus for use in such a method.
Owner:NEKTAR THERAPEUTICS INC
Lyophilization process and products obtained thereby
A lyophilization process which comprises dissolving a material in one or more solvents for said material to form a solution; forcing said material at least partially out of solution by combining the solution and a non-solvent for the material, which non-solvent is miscible with the solvent or solvents used and wherein said non-solvent is volatilizable under freeze-drying conditions. In addition, for hydrophobic and / or lipophilic materials, the anti-solvent can be omitted, and the solution of the material in the solvent can be subjected directly to freeze drying. The lyophilizates can then be reconstituted with typical aqueous diluent in the case of hydrophilic materials. Hydrophobic and or lipophilic materials can be initially reconstituted with propylene glycol and / or polyethyleneglycol to form a high concentration solution therein and this is further diluted for use with a diluent of Intralipid, plasma, serum, or even whole blood.
Owner:SCIDOSE PHARMA +1
Isolation of carotenoid crystals
InactiveUS7015014B2Reduce usageIncrease carotenoid contentOrganic active ingredientsBiocideMicroorganismAnti solvent
The present invention relates to a crystalline carotenoid compound, such as β-carotene, with a purity of at least 95% and with substantially no solvent enclosed in the crystal lattice. The present invention further describes a process to prepare such a highly pure crystalline carotenoid compound from microbial biomass, without the use of a solvent extraction and / or an anti-solvent crystallization process.
Owner:DSM IP ASSETS BV
Hydrodynamic cavitation crystallization process
A device and process for crystallizing a compound using hydrodynamic cavitation comprising the steps of mixing at least one stream of a solution of such compound to be crystallized with at least one stream of an anti-solvent and passing the mixed streams at an elevated pressure through a local constriction of flow to create hydrodynamic cavitation thereby causing nucleation and the direct production of crystals.
Owner:ARISDYNE STSTEMS INC
Hydrodynamic cavitation crystallization device and process
InactiveUS20080194868A1Polycrystalline material growthFrom normal temperature solutionsAnti solventCavitation
A device and process for crystallizing a compound using hydrodynamic cavitation comprising the steps of mixing at least one stream of a solution of such compound to be crystallized with at least one stream of an anti-solvent and passing the mixed streams at an elevated pressure through a local constriction of flow to create hydrodynamic cavitation thereby causing nucleation and the direct production of crystals. The compound to be crystallized can be, for example, an active pharmaceutical ingredient.
Owner:CAVITECH HLDG
Particulate drug-containing products and method of manufacture
InactiveUS7125566B2Improve featuresEfficiently aerosolizedBiocidePowder deliveryParticulatesOrganic solvent
Provided is a compressed anti-solvent technique for manufacture of drug-containing powders for pulmonary delivery. The drug is processed in a cosolvent system including two or more mutually soluble organic solvents. Also provided are powders manufacturable by the manufacture method, including powders of substantially pure drug and powders including a biocompatible polymer for pulmonary sustained drug release applications. Also provided are packaged products including drug-containing powder in a container that is receivable by and operable with a dry powder inhaler to produce an aerosol including dispersed drug-containing particles when the inhaler is actuated.
Owner:ENDO PHARMA COLORADO
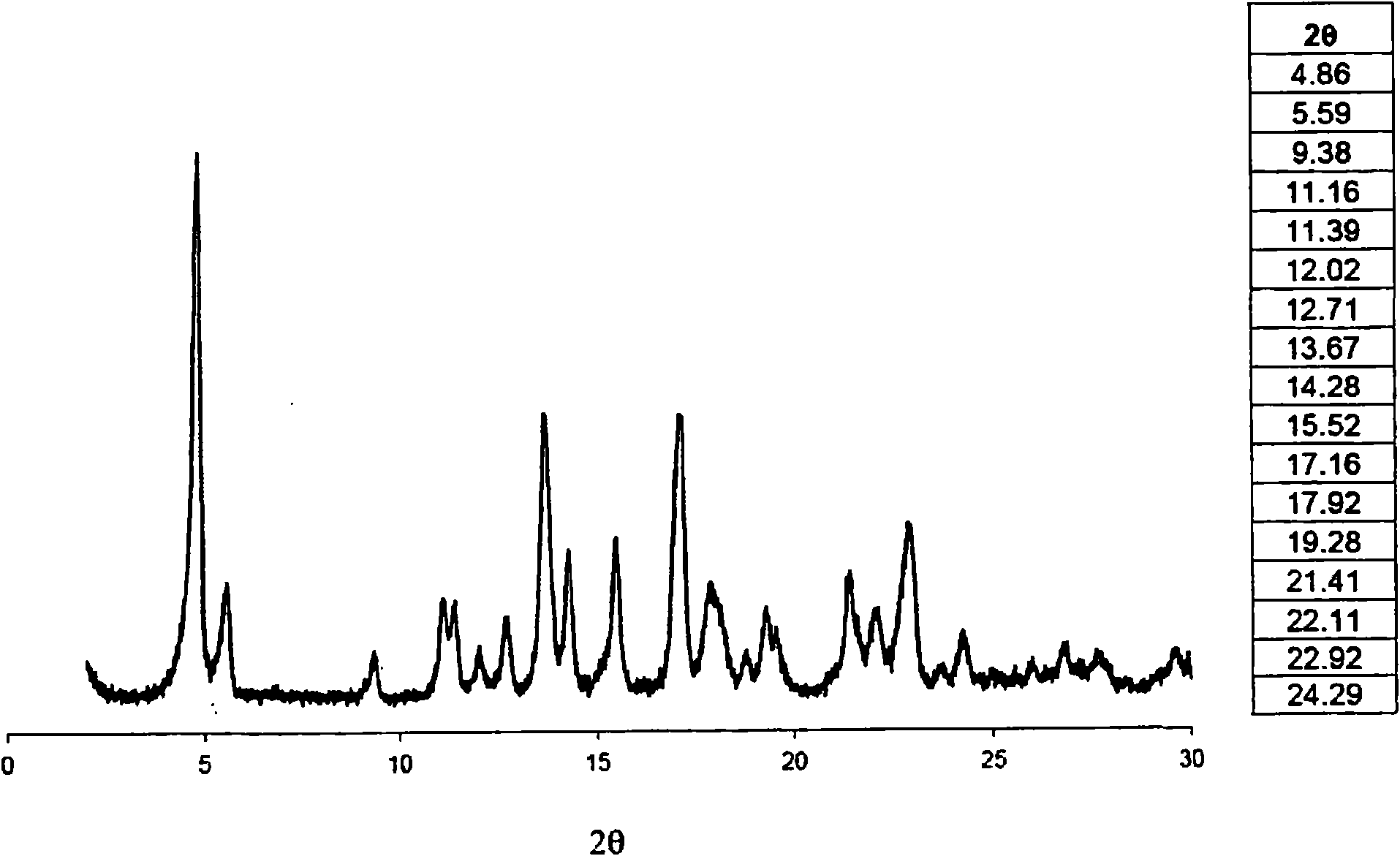
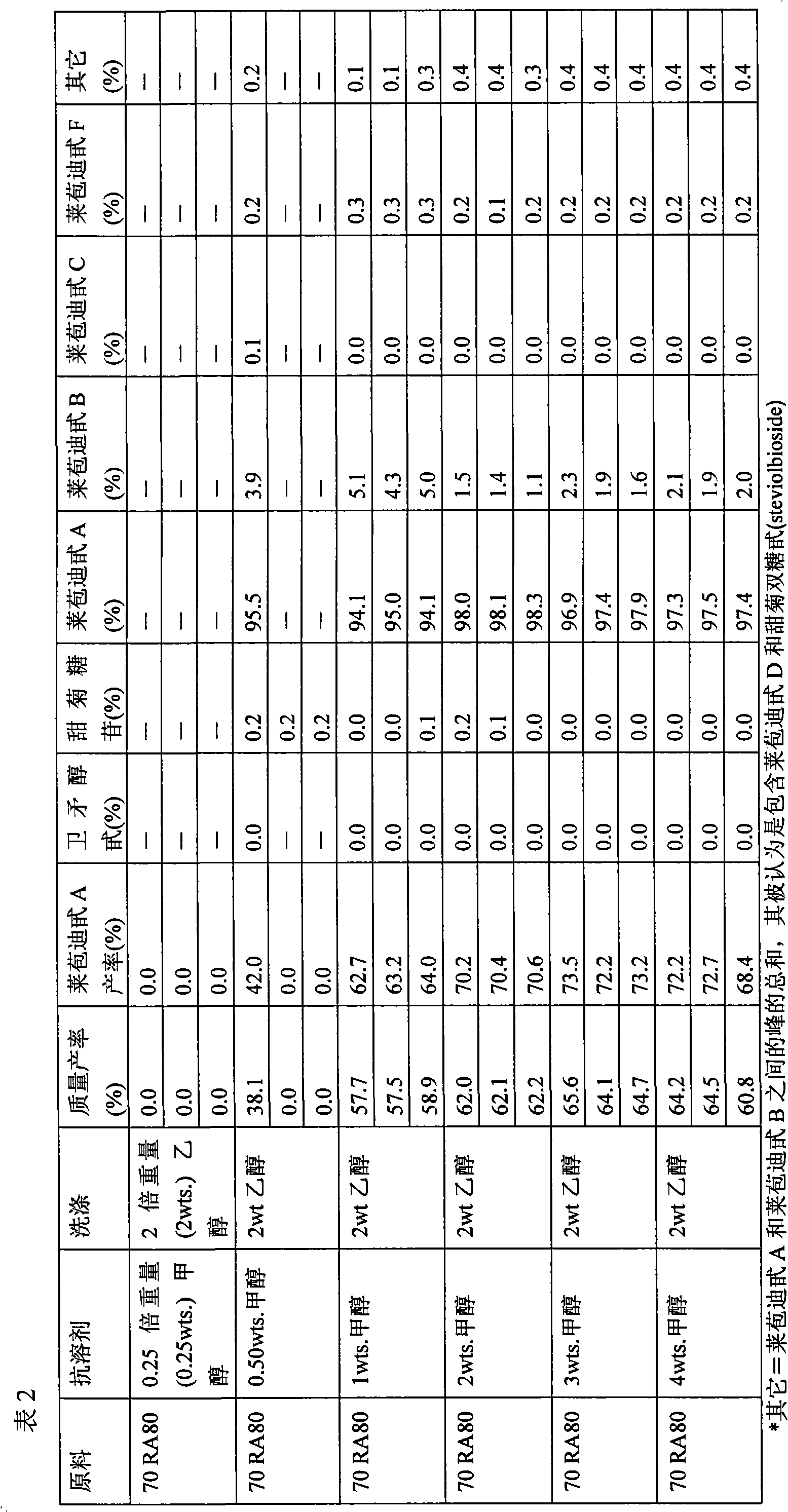
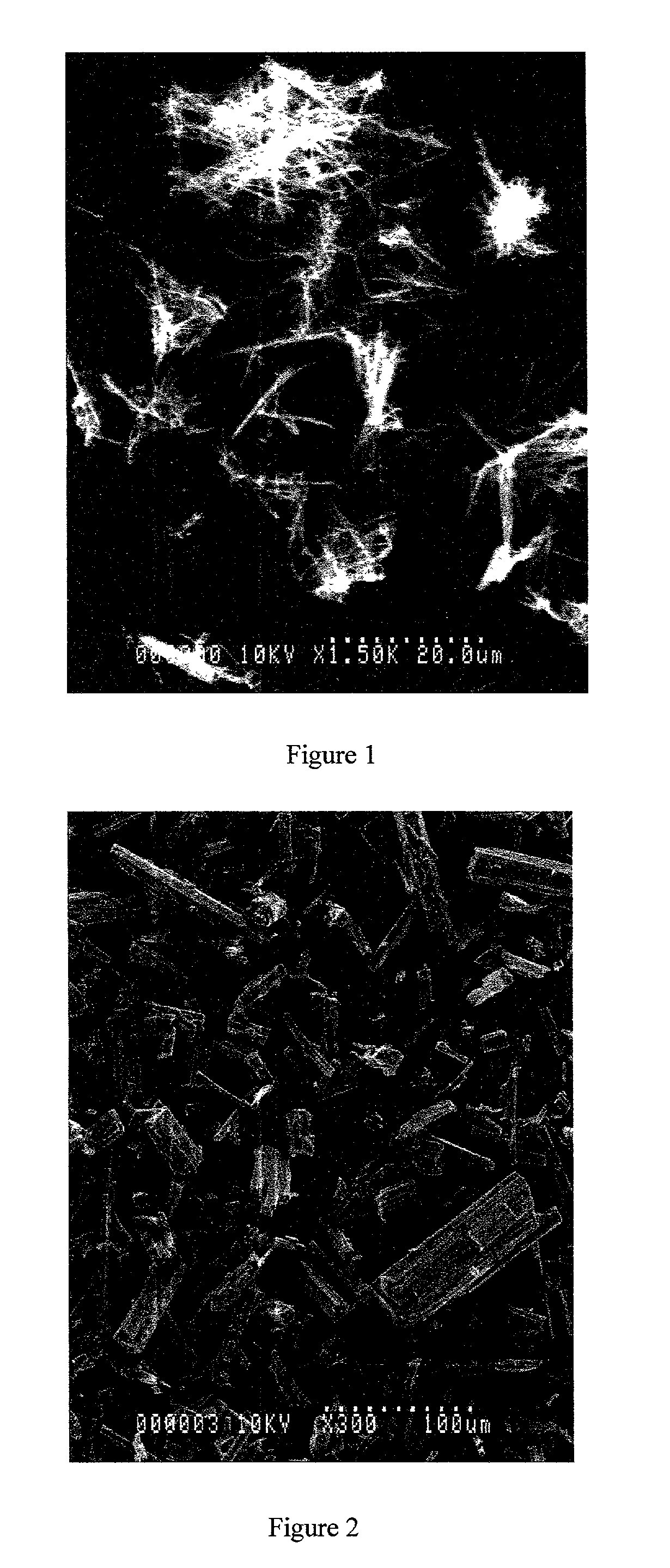
Method of producing purified rebaudioside a compositions using solvent/antisolvent crystallization
The invention provides methods of purifying rebaudioside A from a mixture comprising glycosides of the plant Stevia rebaudiana. The methods of the invention are useful for preparing highly pure rebaudioside A compositions from crude Stevia starting compositions that are typically considerably lower in rebaudioside A concentration. The highly pure rebaudioside A compositions are useful as non-caloric sweeteners in edible or chewable compositions such as food, beverages, medicine, candy, chewing gum, and the like.
Owner:CARGILL INC
Particle formation methods and their products
Preparation of particles of an active substance having a layer of an additive at the particle surfaces, by dissolving both the active substance and the additive in a vehicle to form a target solution, and contacting the target solution with an anti-solvent fluid using a SEDS(TM) particle formation process, to cause the active substance and additive to coprecipitate. The additive is typically a protective additive, in particular a taste and / or odour masking agent. Also provided is a particulate coformulation made by the method, which has a finite gradient in the relative additive concentration, which concentration increases radially outwards from the active-rich core to the additive-rich surface of the particles.
Owner:NEKTAR THERAPEUTICS INC
Technology for the Preparation of Microparticles
InactiveUS20090098207A1Promote formationImprove stabilityPowder deliveryOrganic active ingredientsAnti solventMicrosphere
Microspheres are produced by contacting a solution of a macromolecule or small molecule in a solvent with an antisolvent and a counterion, and chilling the solution. The microspheres are useful for preparing pharmaceuticals, nutraceuticals, cosmetic products and the like of defined dimensions.
Owner:NEXBIO INC
Method of particle formation
InactiveUS20050206023A1Characteristic is differentSufficient flow ratePressurized chemical processPeptide/protein ingredientsAnti solventTemperature and pressure
The invention provides a method for forming particles of a target substance, comprising (a) co-introducing into a particle formation vessel, under controlled temperature and pressure, a supercritical or near-critical anti-solvent fluid; a “target solution or suspension” of the target in a first vehicle; and a second vehicle which is soluble in the anti-solvent fluid; and (b) using the anti-solvent to disperse the target solution / suspension and the second vehicle, and to extract the vehicles, substantially simultaneously and substantially immediately on introduction of the fluids into the particle formation vessel, wherein the second vehicle is immiscible with the first, and wherein contact between the target solution / suspension and the second vehicle occurs a sufficiently short period of time before their dispersion by the anti-solvent, and with sufficient physical mixing, as to allow only insignificant, if any, phase separation to occur between the two vehicles between their contact with one another and their dispersion.
Owner:NEKTAR THERAPEUTICS INC
Supercritical fluids processing: preparation of protein microparticles and their stablilisation
InactiveUS7250152B2Improve solubilityIn-vivo radioactive preparationsEnzyme stabilisationAnti solventMicroparticle
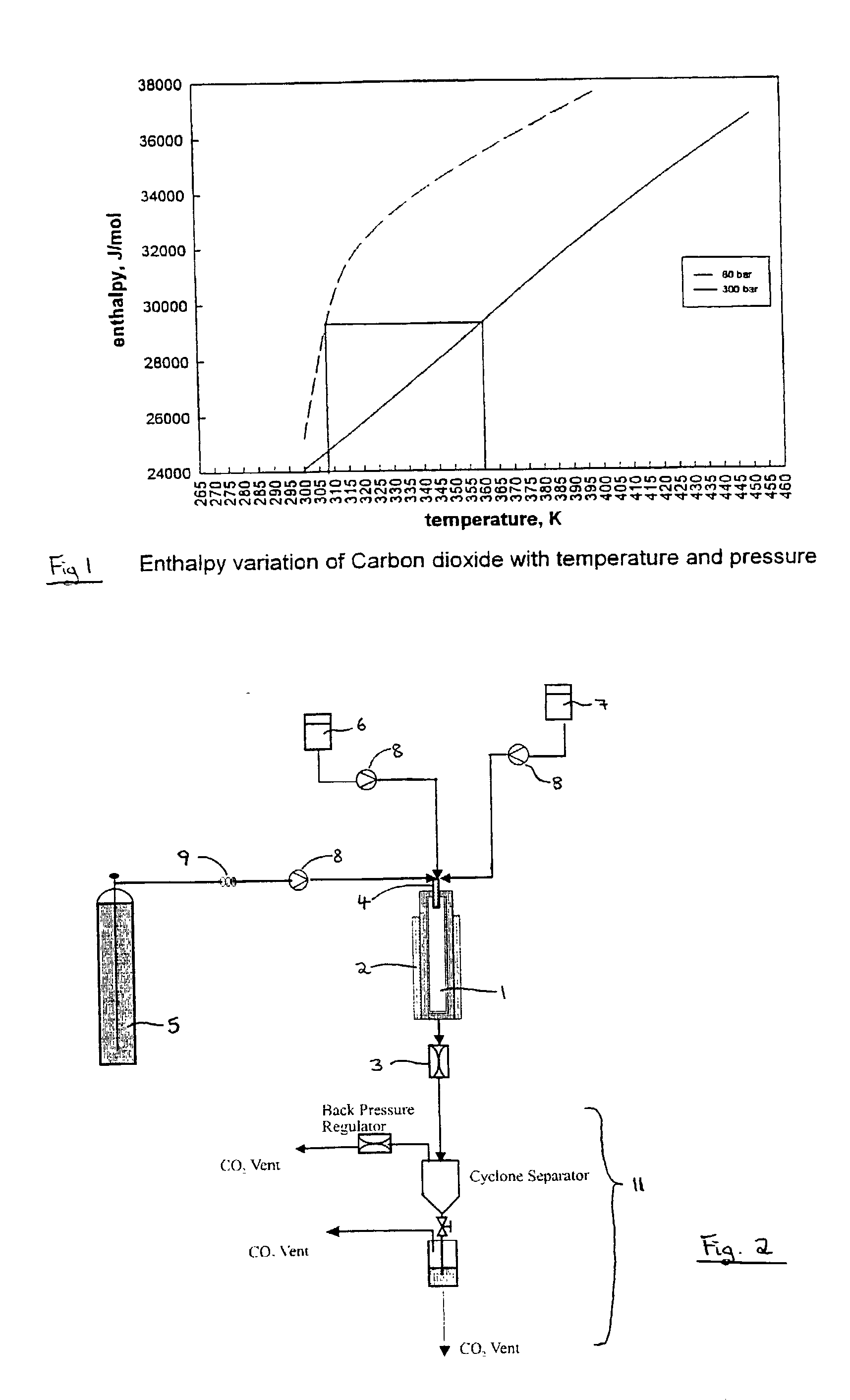
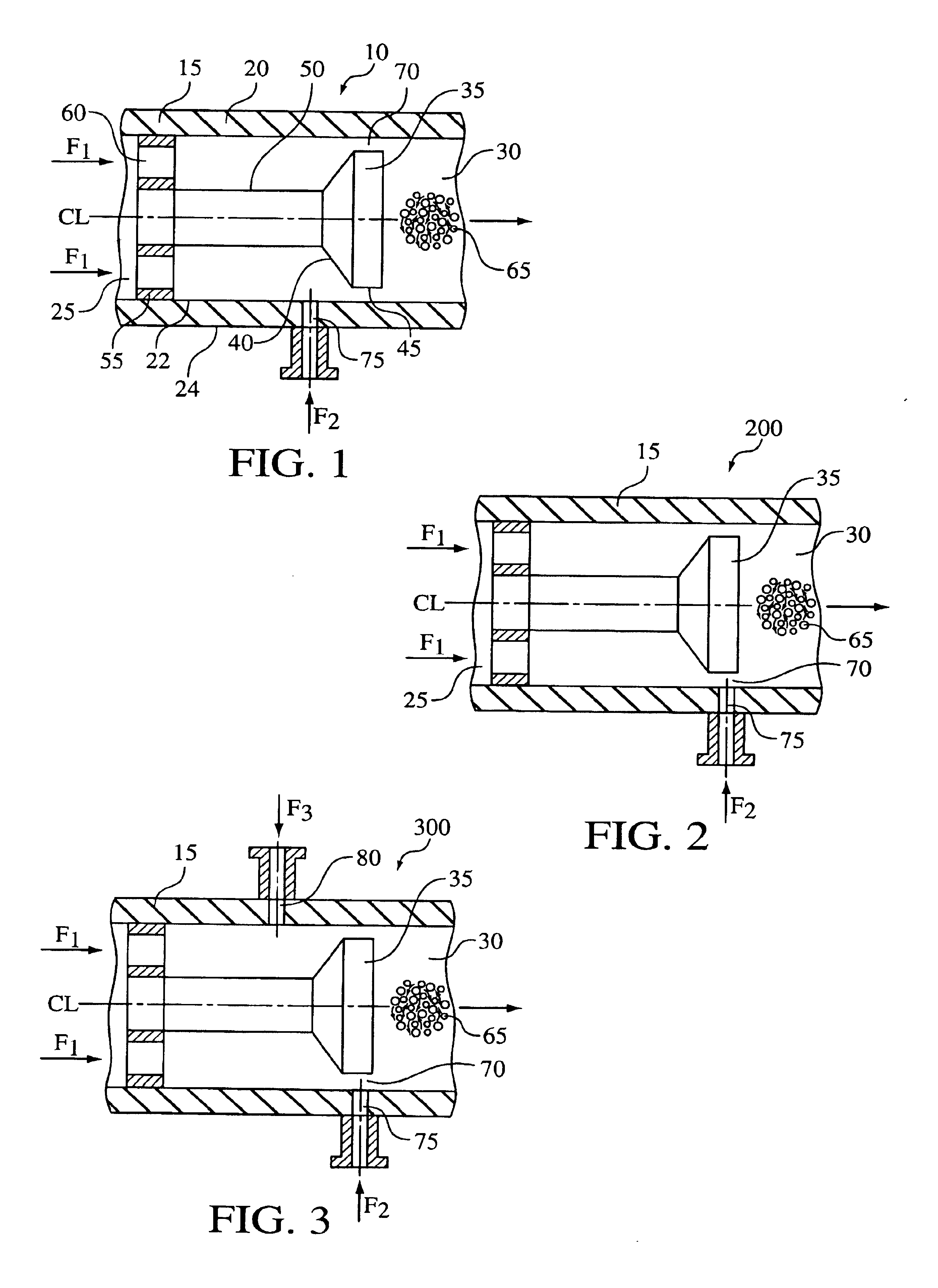
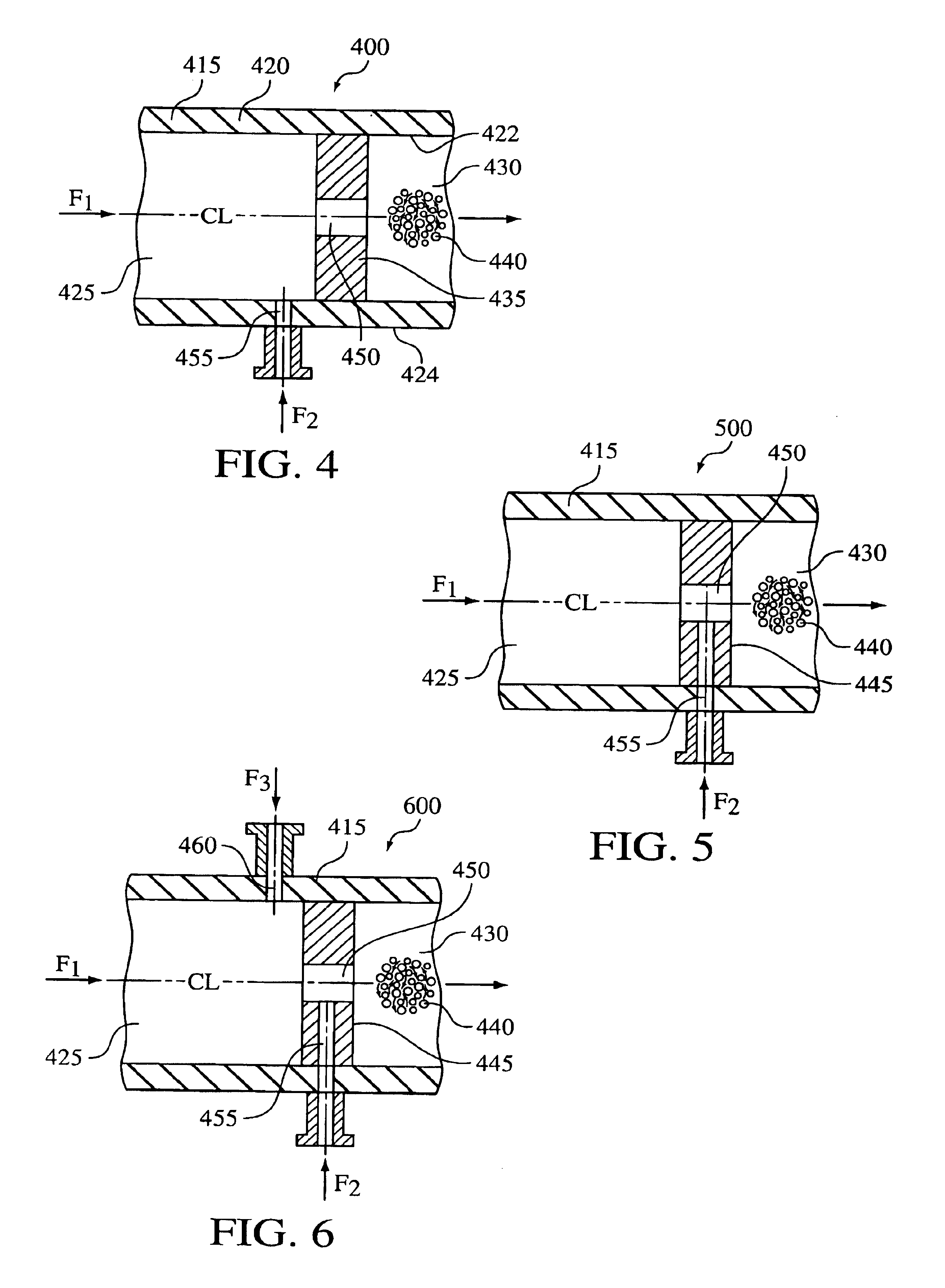
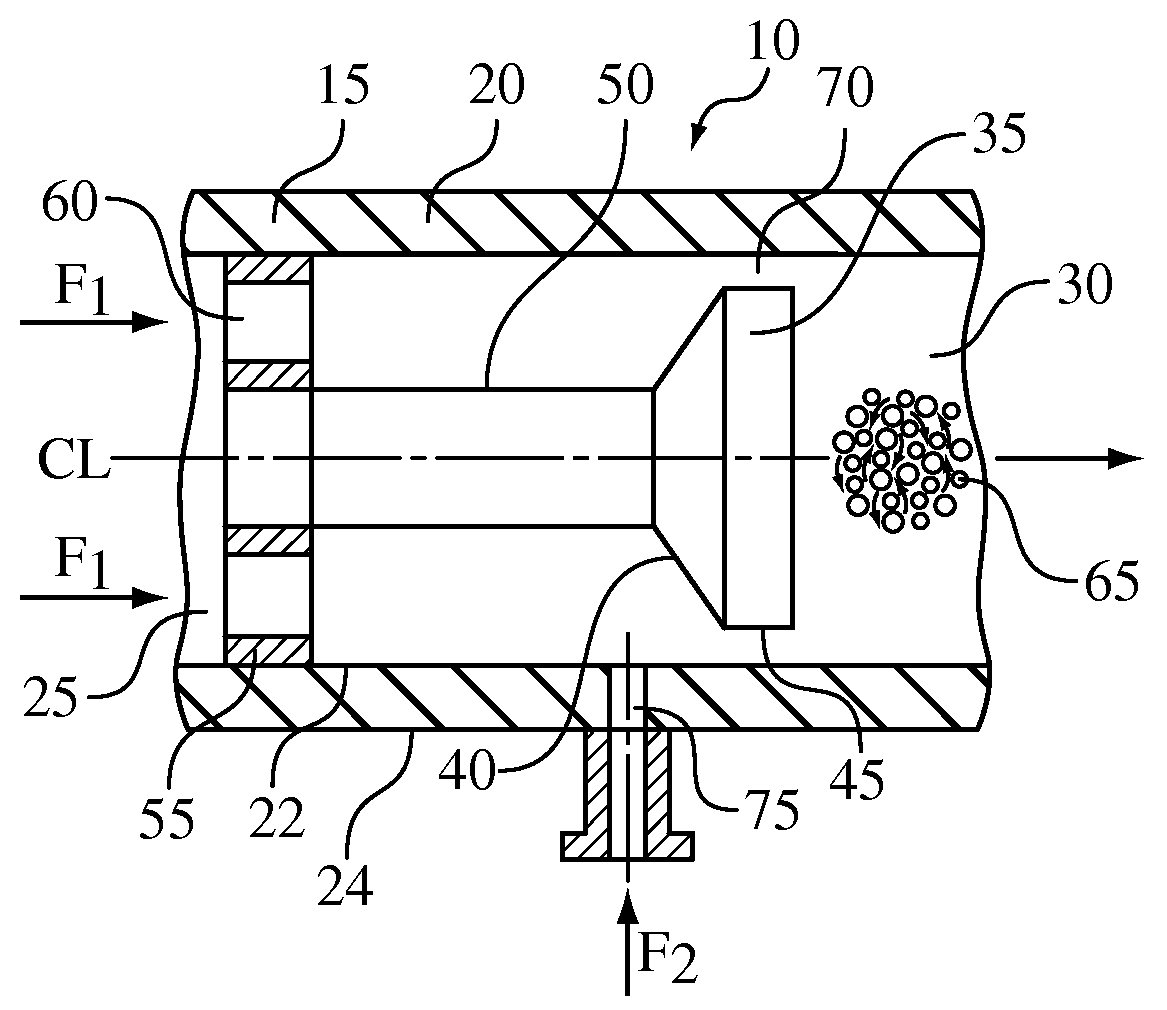
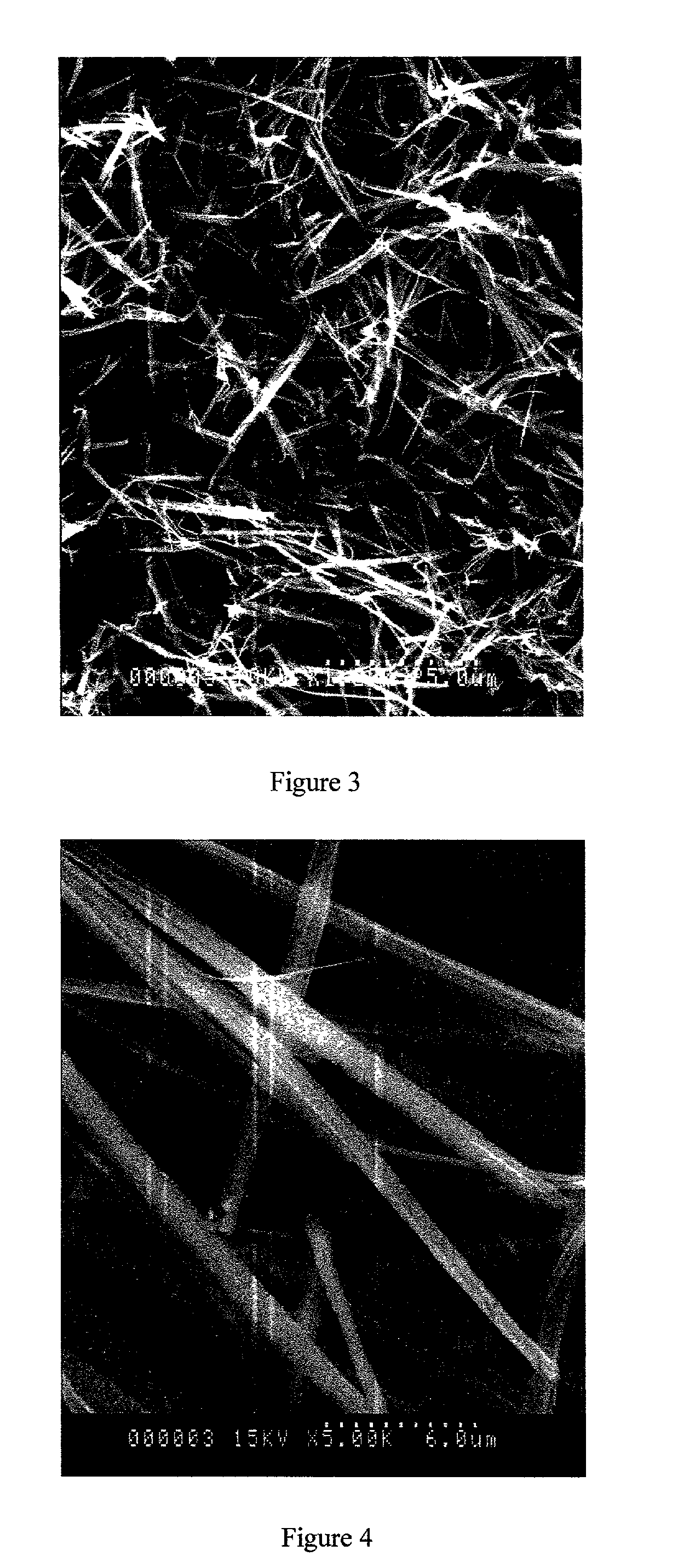
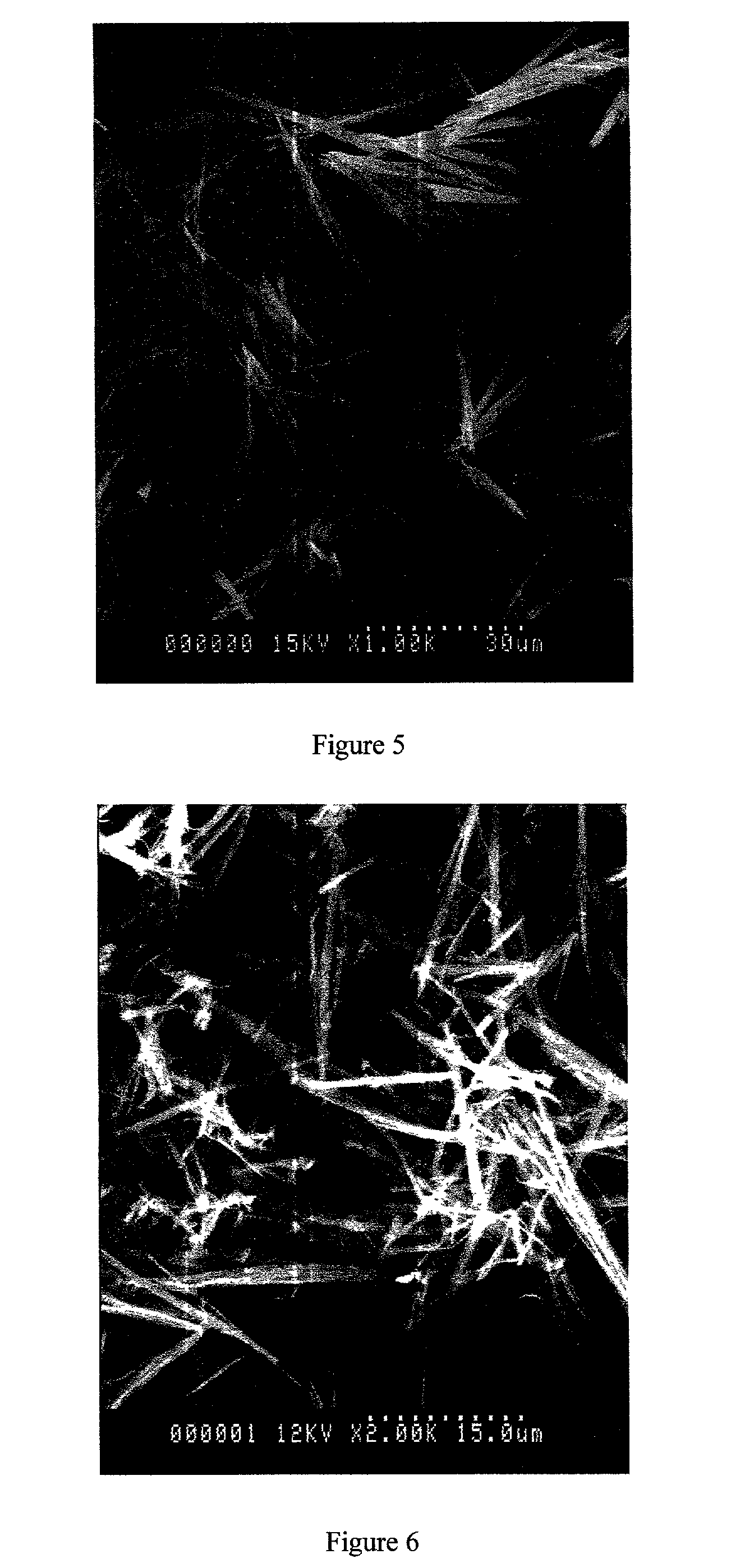
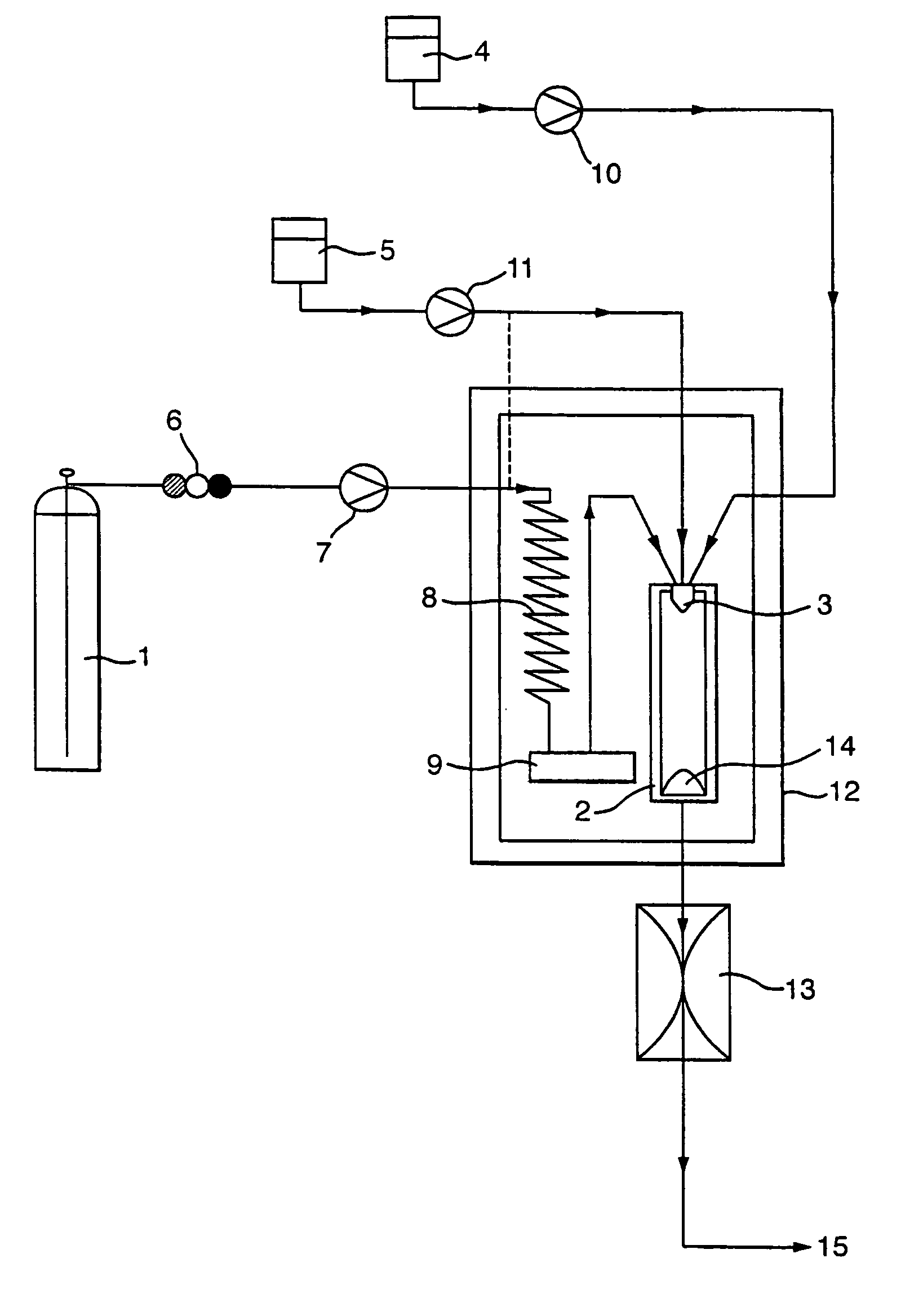
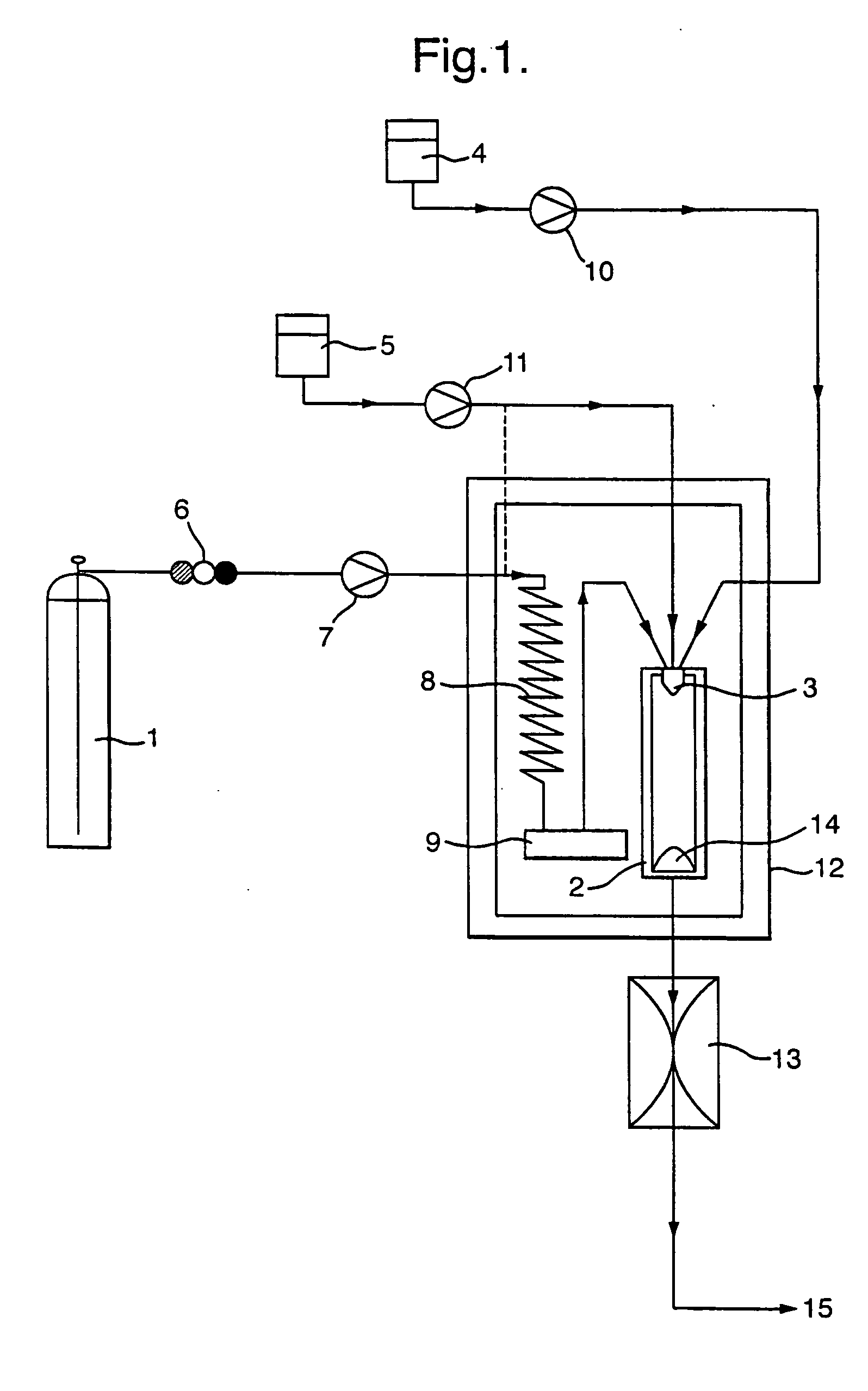
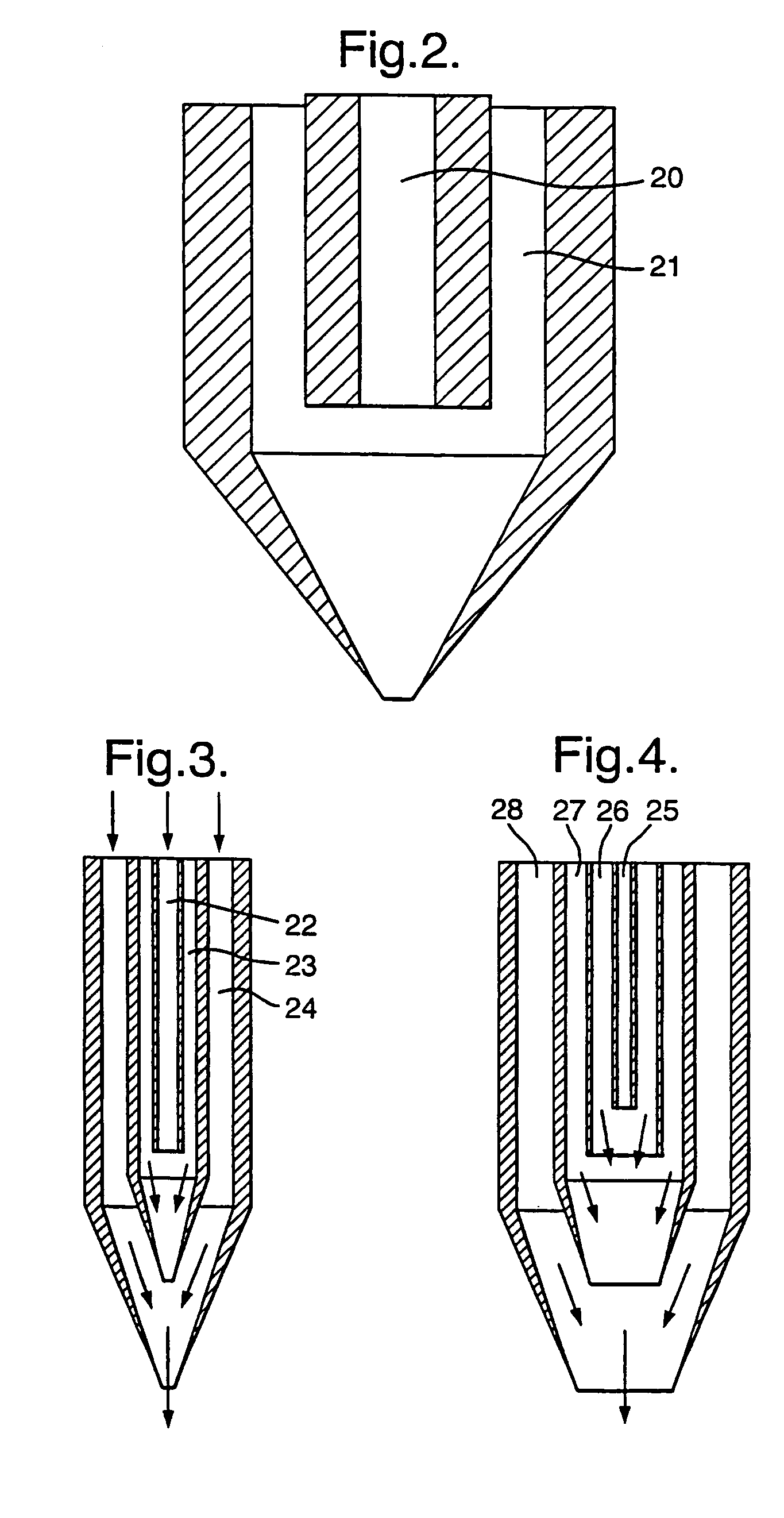
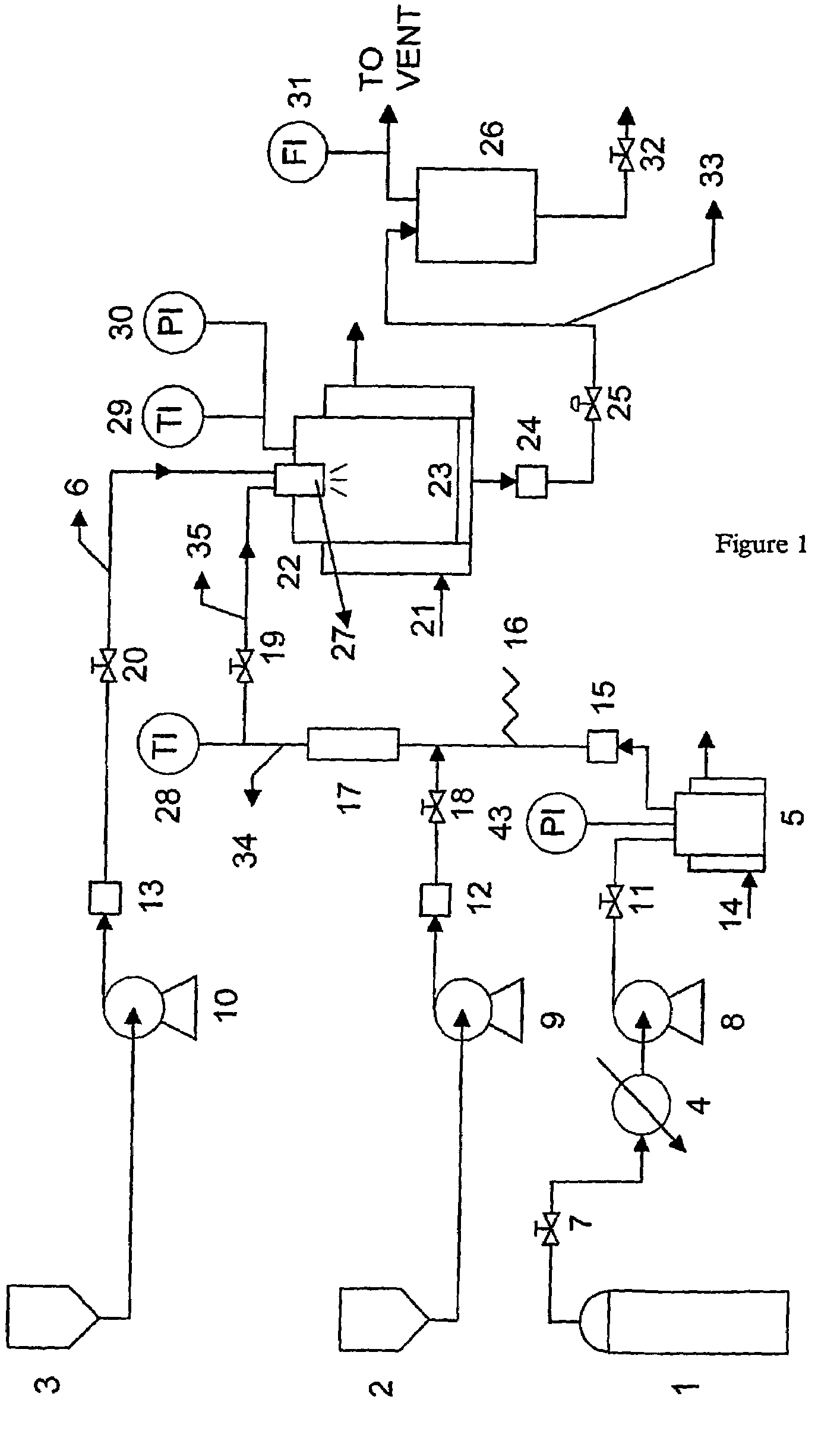
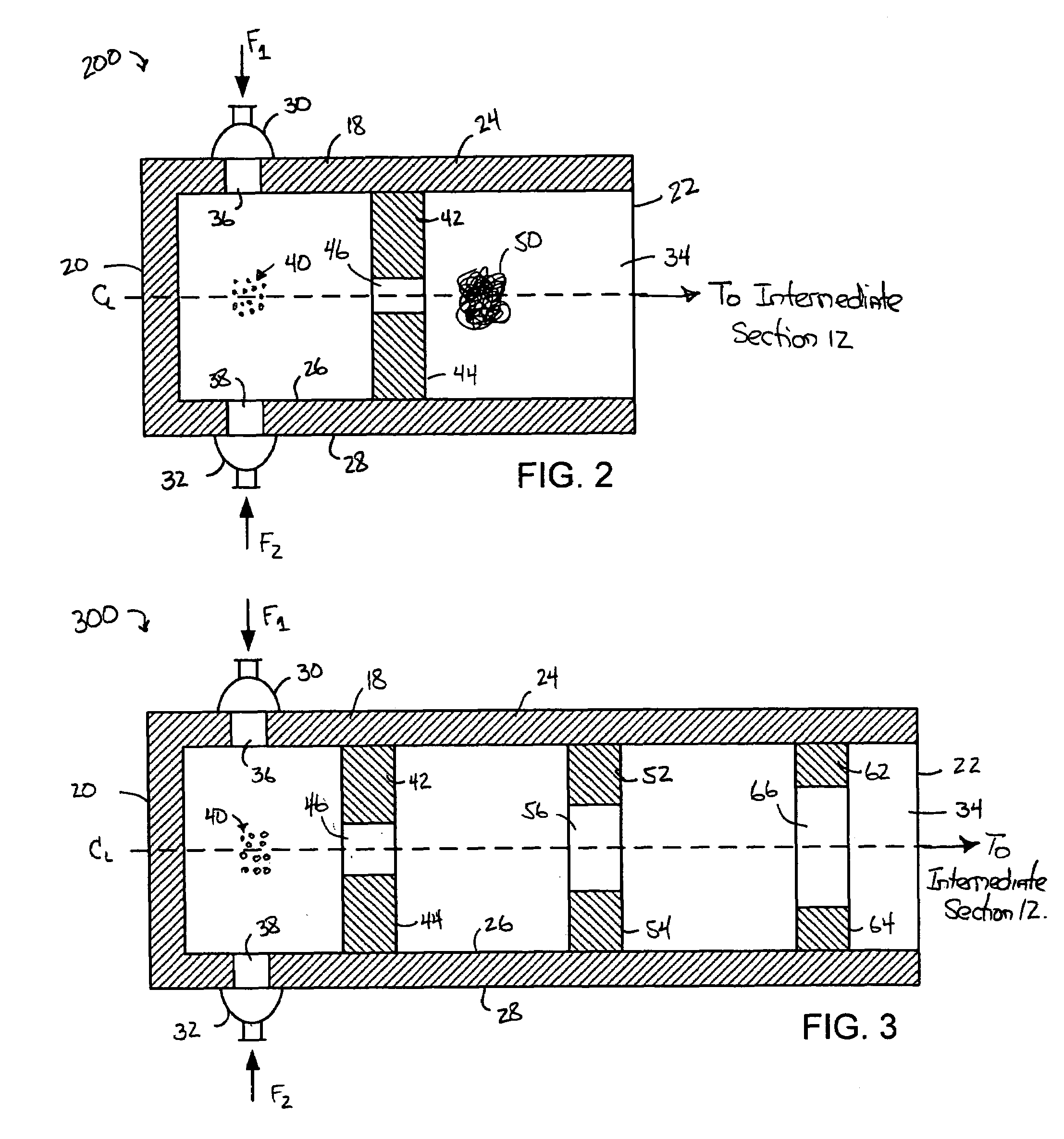
A process for the co-precipitation of a substance with a stabilizer therefor, by a gas anti solvent process comprising introducing into a particle formation vessel a supercritical fluid pure or mixed with a modifier; and a solution comprising said substance and said stabilizer dissolved in a solvent; so as said solvent is extracted from the solution by said supercritical fluid and co-precipitation of the substance and stabilizer occurs. The process may be carried out using an apparatus, for example, shown in FIG. 1, comprising a particle formation vessel (22) and a nozzle (27) having a central orifice (39) serving to introduce a solution of the substance and a plurality of outer orifices (41) serving to carry a flow of supercritical fluid into the particle formation vessel (22), such that the solvent is extracted from the solution by the supercritical fluid and precipitation of micron sized particles of the substance / stabilizer occurs.
Owner:DOMPE SPA +1
Perovskite membrane and preparation and application method thereof
InactiveCN104022185AImprove flatnessGood solvent resistanceFinal product manufacturePhotovoltaic energy generationAnti solventCharge carrier
The invention belongs to the technical field of solar cells and particularly relates to a perovskite membrane and a preparation and application method of the perovskite membrane. The perovskite membrane is generated through a two-step method, a PbI2 membrane is prepared through a solution processing method, and thus the technology is simple and efficient. Besides, due to the two-step method, the flatness of the surface of the perovskite membrane is effectively improved, recombination of carriers on the active layer interface is greatly reduced, the anti-solvent performance of the material is effectively improved, and the performance of a device is obviously improved. The perovskite membrane further has the advantages of being simple in preparation technology, low in cost, good in experimental repeatability, suitable for large-scale industrial production and the like.
Owner:NORTH CHINA ELECTRIC POWER UNIV (BAODING)
Method for preparing stable albumin nanoparticle
InactiveCN102988996AProcess stabilityPowder deliveryLyophilised deliveryRedox responsiveDisulfide bond
The invention relates to a method for preparing a stable albumin nanoparticle, and belongs to the technical field of preparation of biomedical materials. The method comprises the following steps: pretreating albumin by using glutathione and cysteine without biotoxicity; opening an intramolecular disulfide bond; precipitating the albumin by using anti-solvents such as alcohol and the like; and carrying out exchange reaction on a sulfydryl-disulfide bond to obtain the albumin nanoparticle containing the intramolecular disulfide bond. The prepared albumin nanoparticle can be used for the delivery of pharmacological active substances and / or diagnostic agents in an organism. The albumin nanoparticle provided by the invention has the advantages that the albumin nanoparticle has good stability under a dilution condition and gives an oxidation reduction response in a reduced environment. Based on the characteristics, the albumin nanoparticle can stably exist in a blood circulation system of the organism, and can carry out a degradation reaction in a cell under the action of reduced glutathione so as to release a wrapped medicine.
Owner:TSINGHUA UNIV
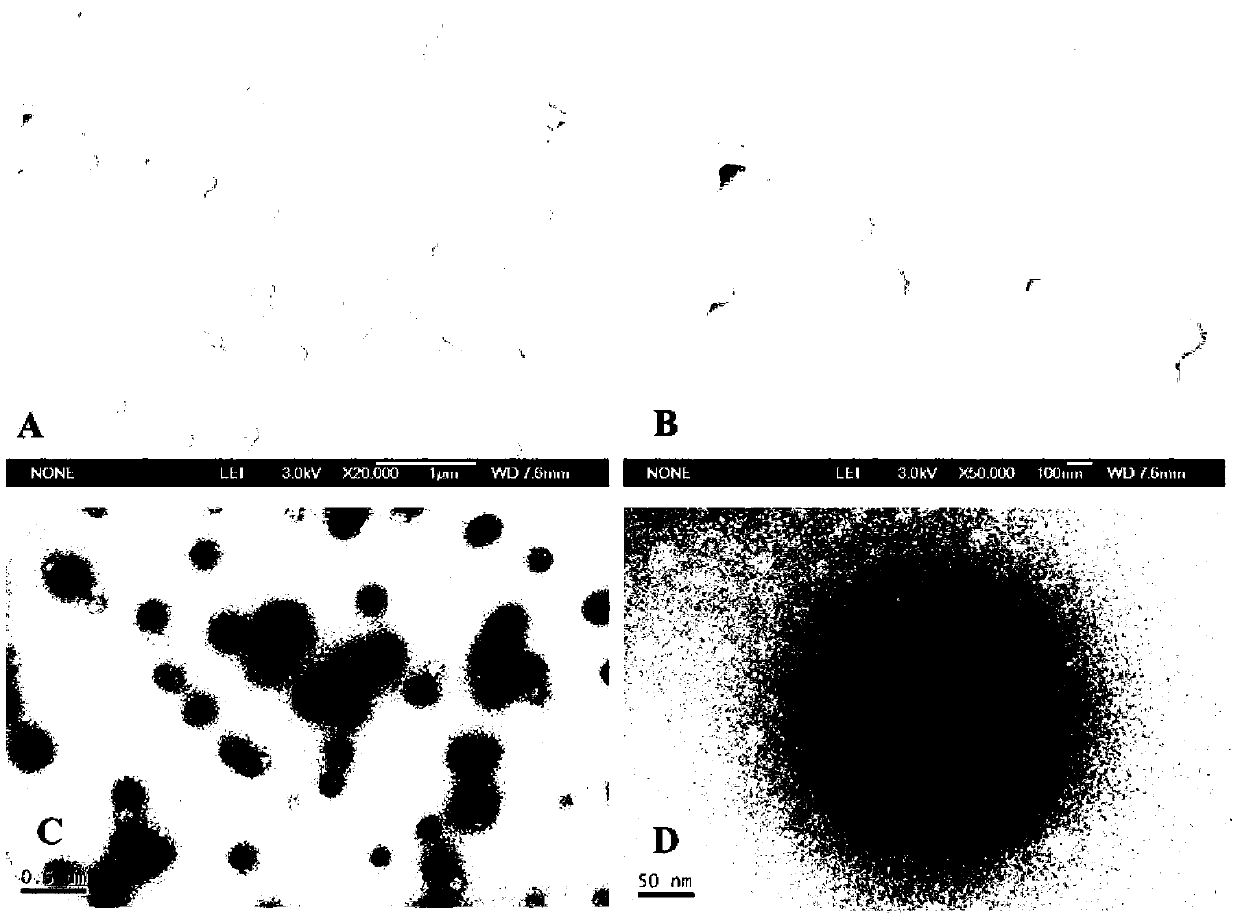
Large-area perovskite film and perovskite solar cell or module and fabrication method thereof
InactiveUS20170287648A1Quality improvementUniform and high-qualityLight-sensitive devicesSolid-state devicesAnti solventPerovskite solar cell
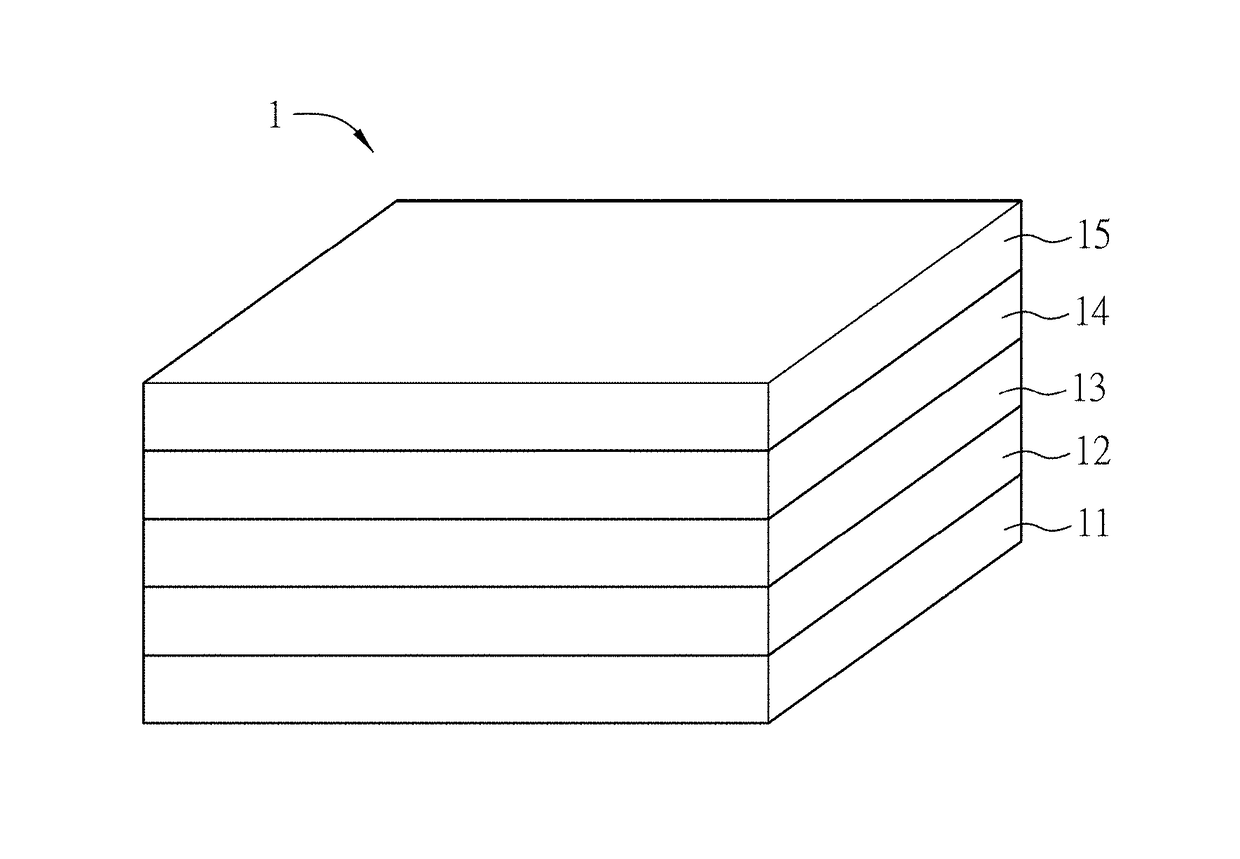
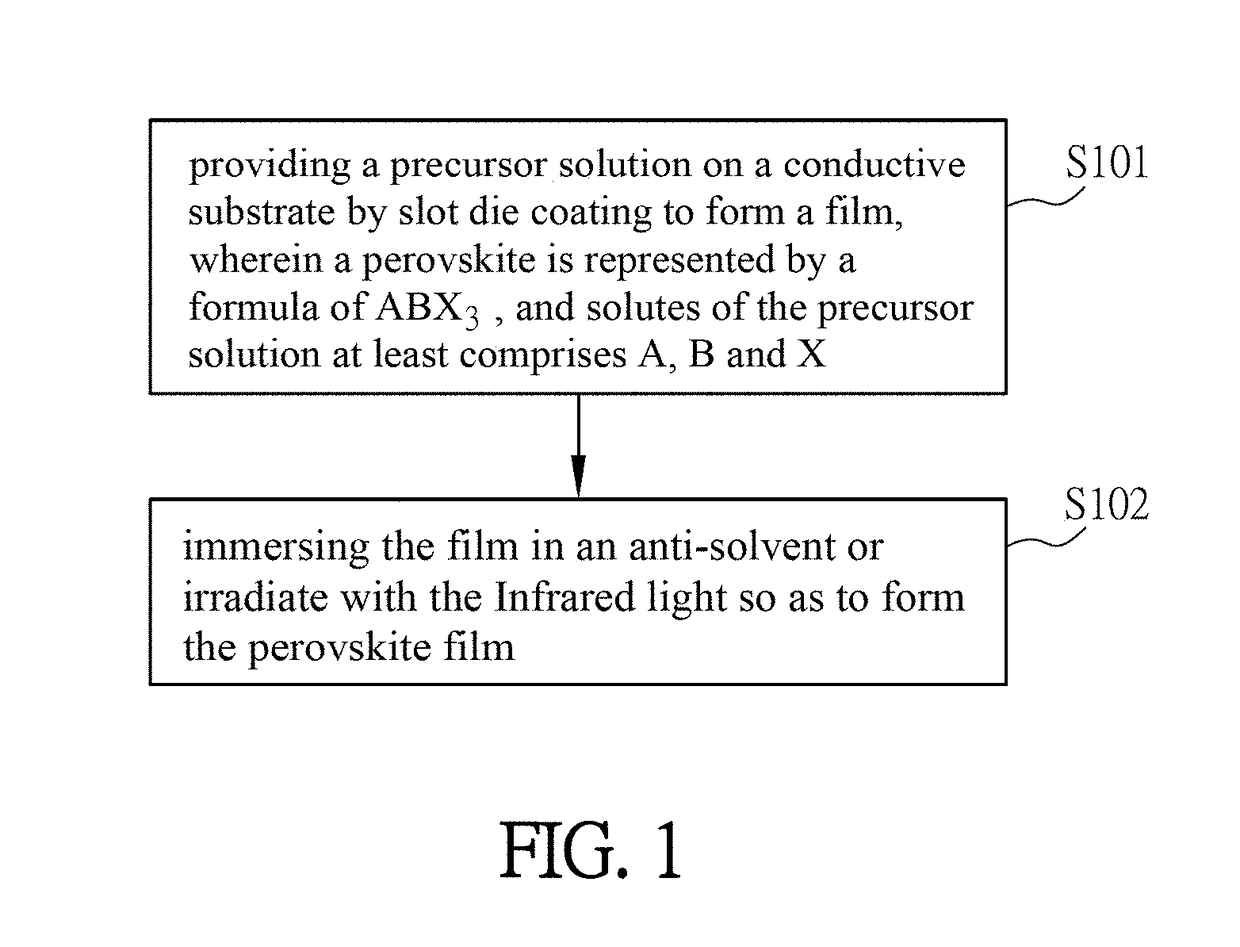
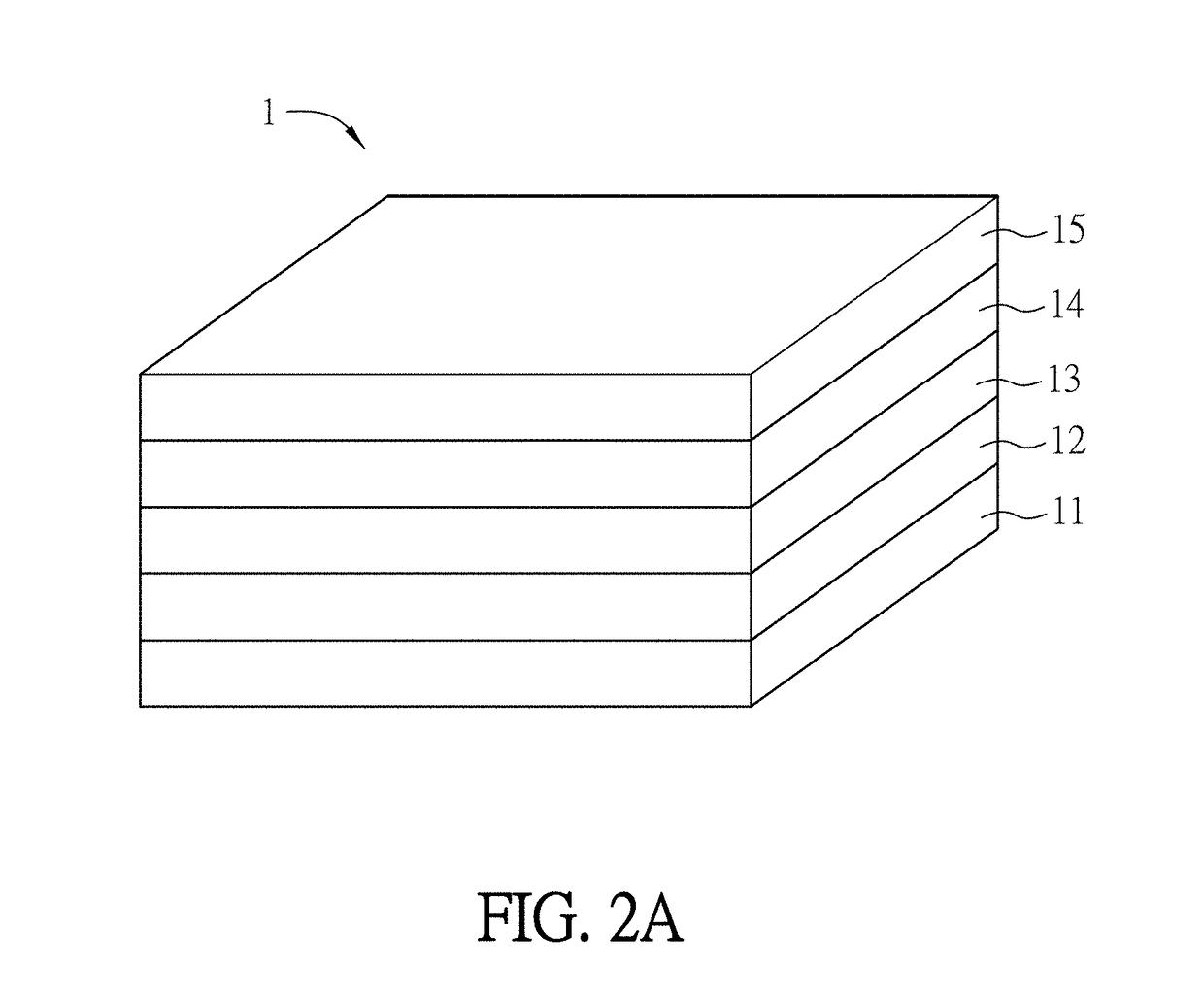
A method of fabricating a large-area perovskite film includes steps of: providing a precursor solution on a conductive substrate to form a film, wherein the perovskite is represented by a formula of ABX3, and the solutes of the precursor solution at least comprises A, B and X; and applying an anti-solvent or Infrared light on the film. The fabrication methods of a large-area perovskite film and a perovskite solar cell or module are also disclosed.
Owner:NAT CENT UNIV
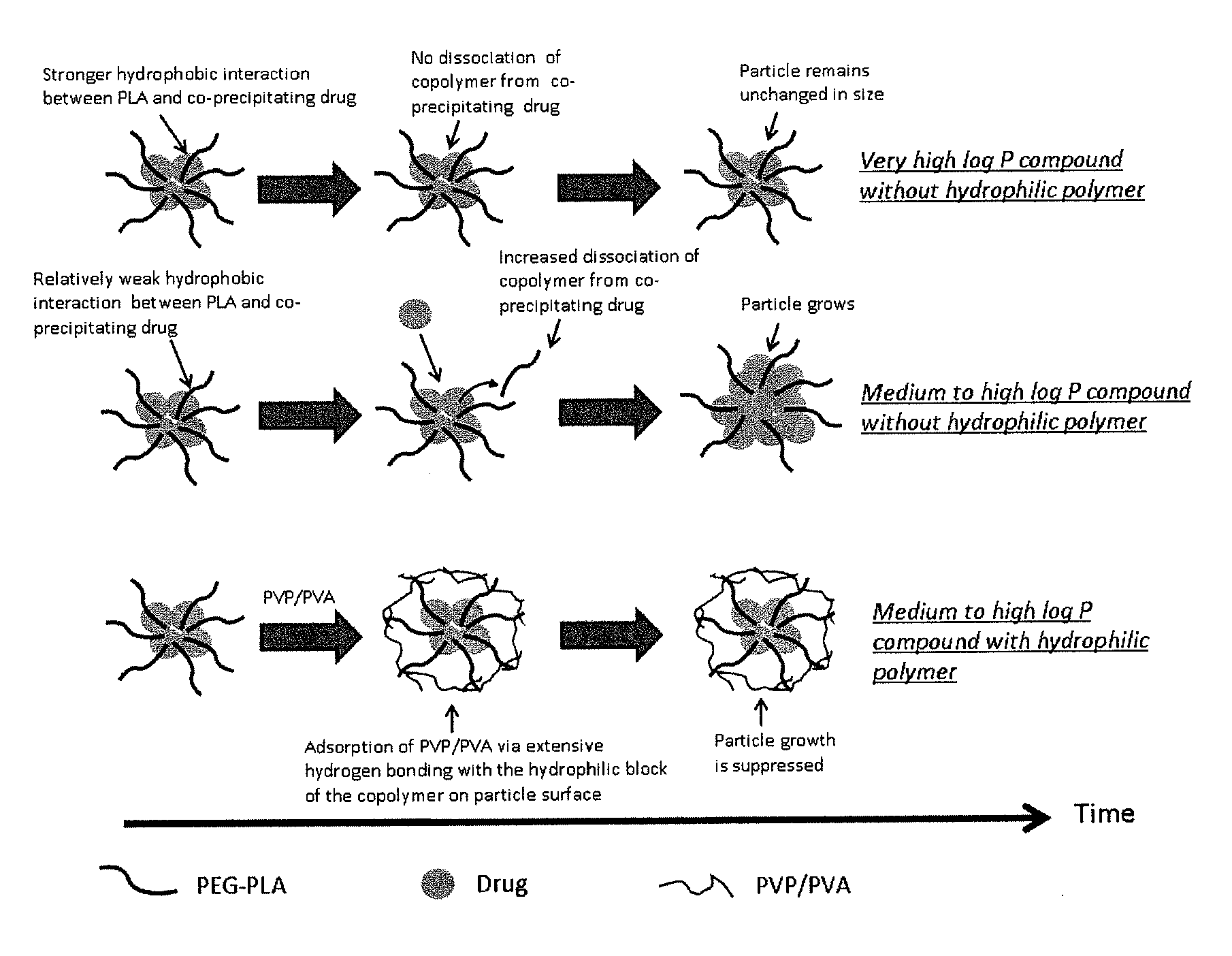
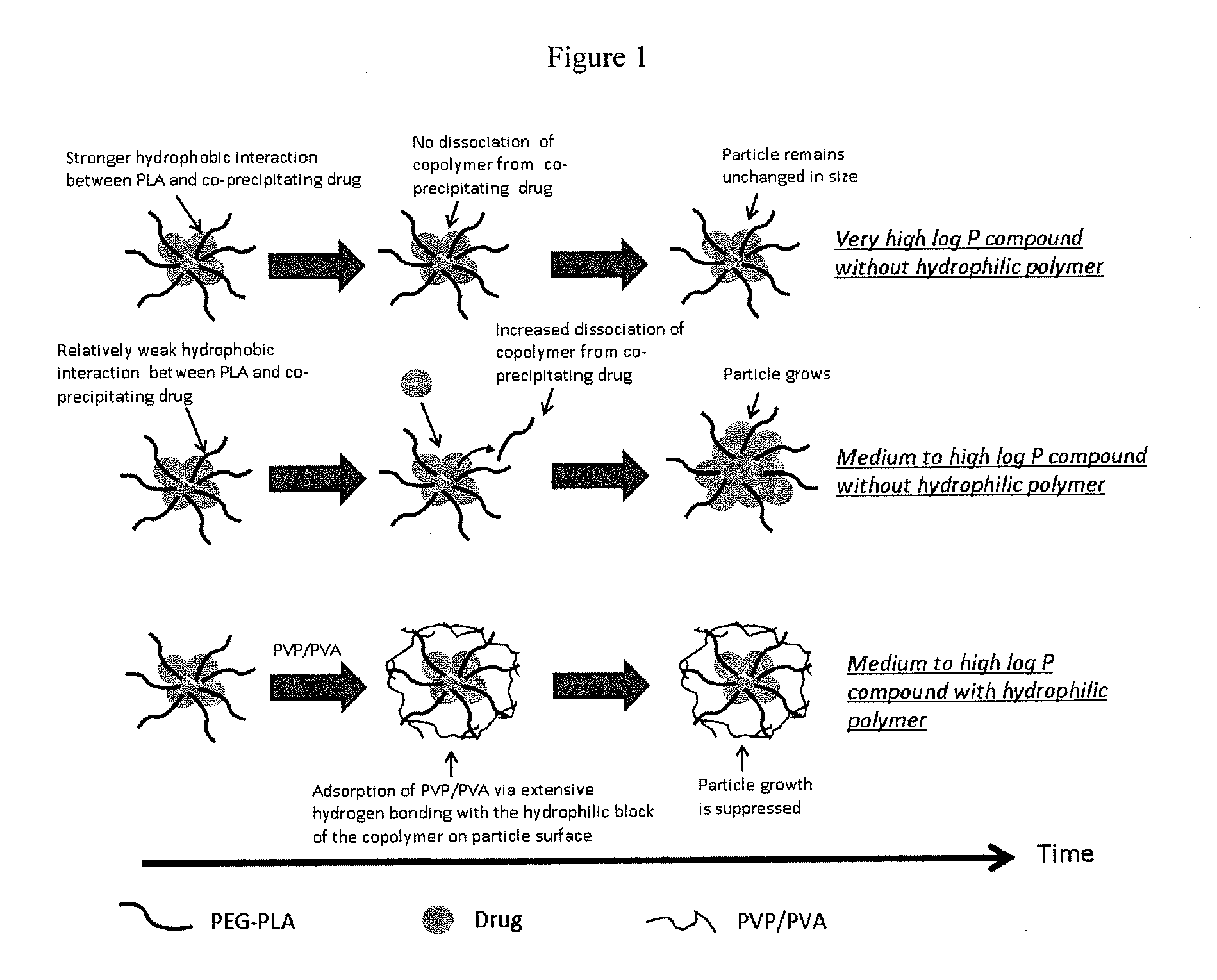
Engineering of polymer-stabilized nanoparticles for drugs with log p values below 6 by controlled antisolvent precipitation
The present invention provides organic nanoparticles that include a molecule having a Log P value of about 3 or above, an amphiphilic diblock copolymer or a surfactant, and a pharmaceutically-acceptable hydrophilic polymer. The present invention also provides methods of making these nanoparticles, e.g., by flash nanoprecipitation, with control over particle size and surface properties. The methods of the present invention provide a means for co-precipitating a water-insoluble compound with an amphiphilic stabilizer within a few milliseconds. The nanoparticles of the present invention exhibit high drug loading, e.g., 50% w / w, and can be produced with a mean particle size less than 200 nm and with a narrow particle size distribution.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
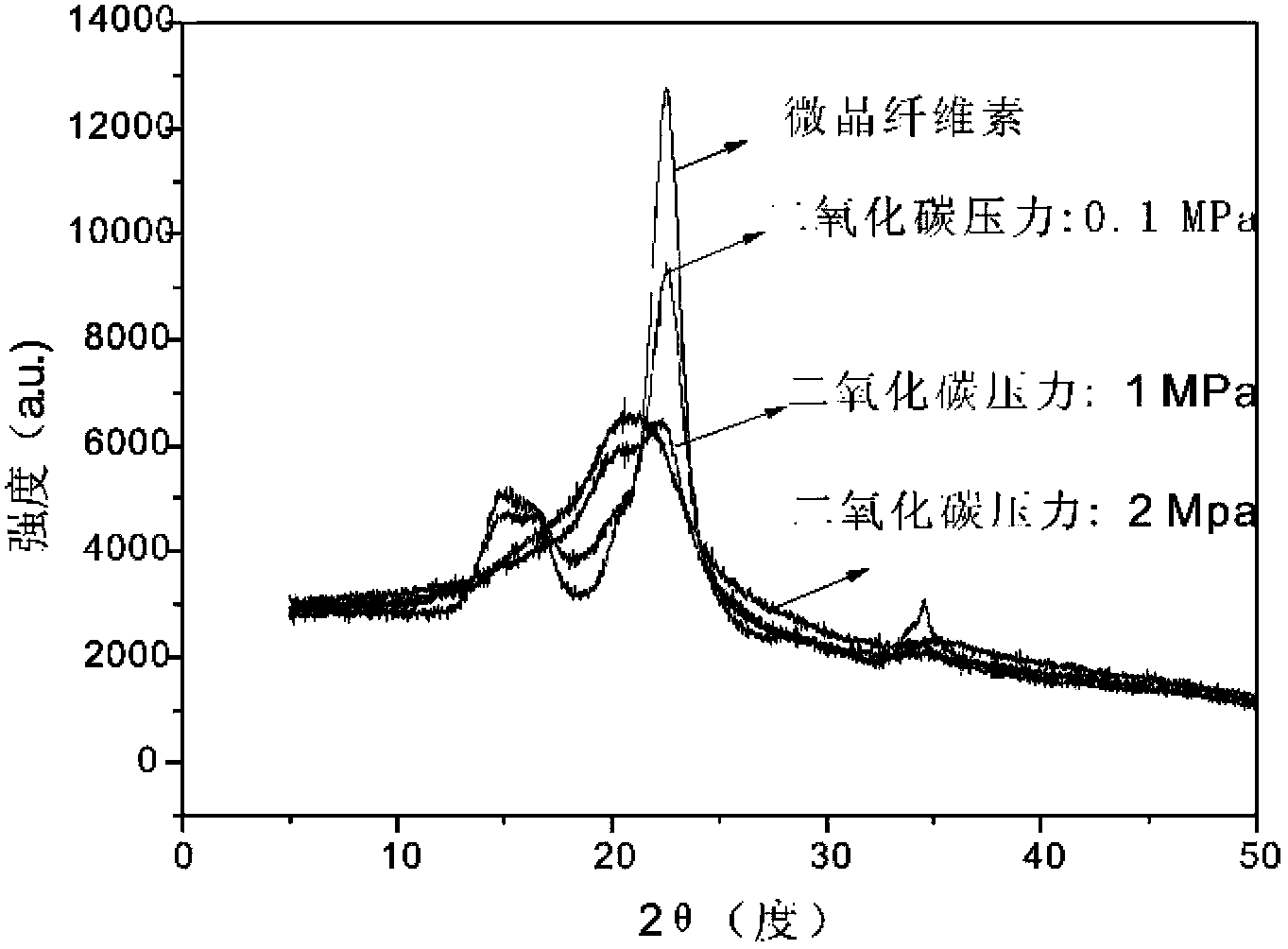
Cellulose solution, cellulose dissolution method and regenerated cellulose
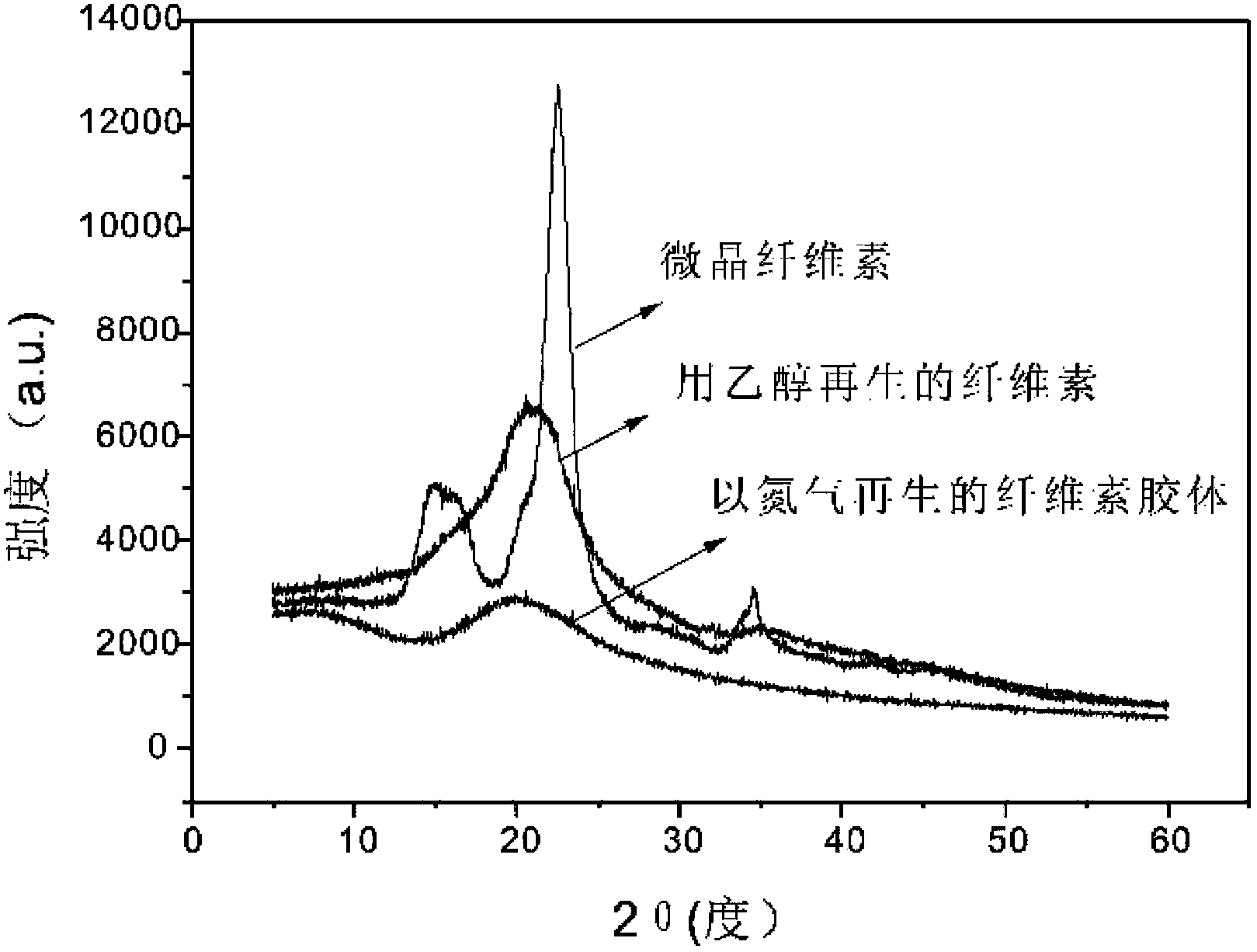
The invention relates to a method for dissolving cellulose in a CO2 switchable ionic compound or a mixed system composed of a CO2 switchable ionic compound and an organic solvent. Various anti-solvents are added into the system, or a gas is introduced into the system or heating is conducted to destroy the structure of a CO2 switchable solvent. The dissolved cellulose regenerates in various physical structure forms. By adjusting the CO2 pressure, and selecting organic amine or alcohol of cations and anions able to compose a CO2 switchable ionic compound or the structure of an assistant organic solvent, the solubility of cellulose and various properties of the solution can be controlled.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Hydrodynamic cavitation crystallization device and process
InactiveUS7314516B2Easily appearPolycrystalline material growthFrom normal temperature solutionsCavitationAnti solvent
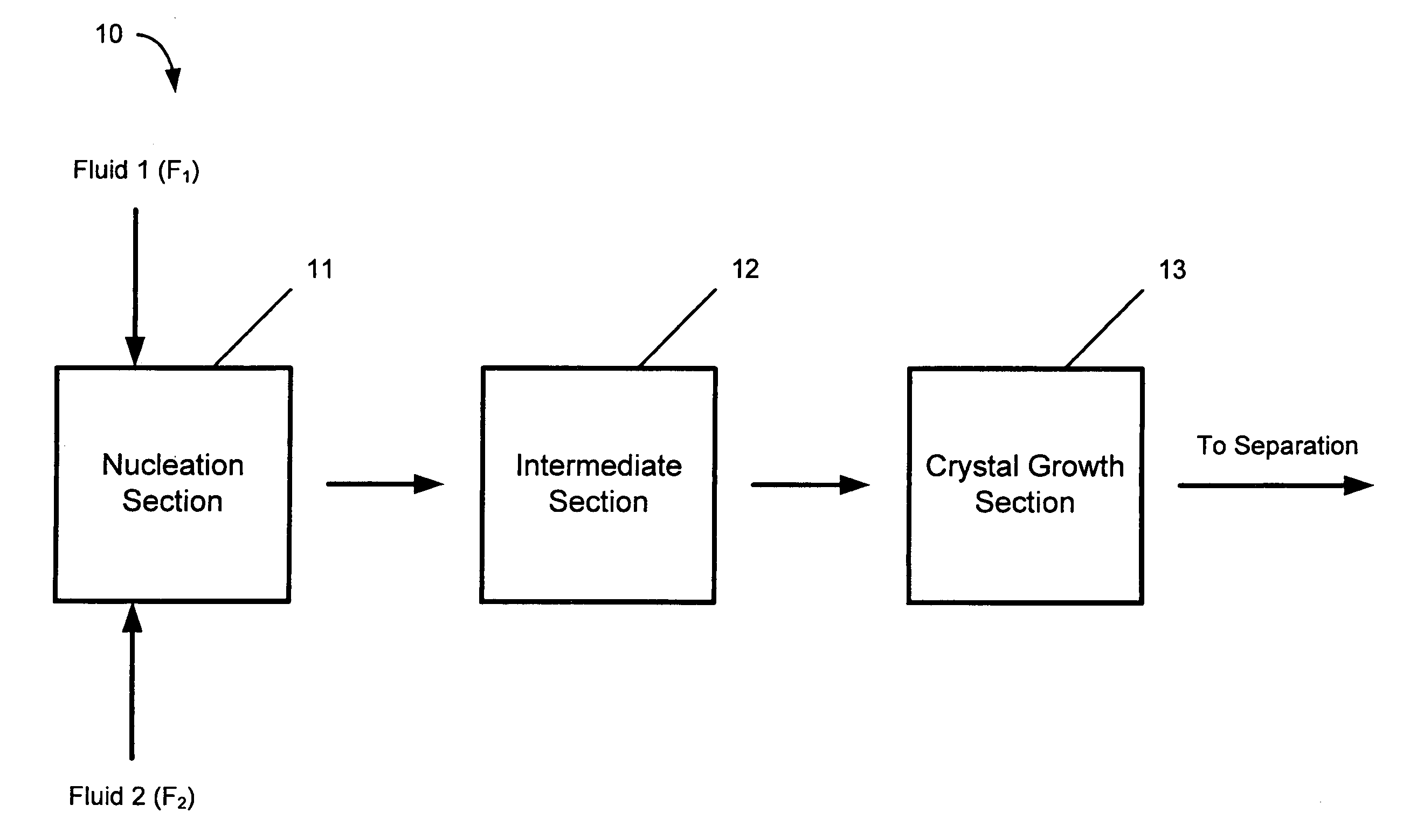
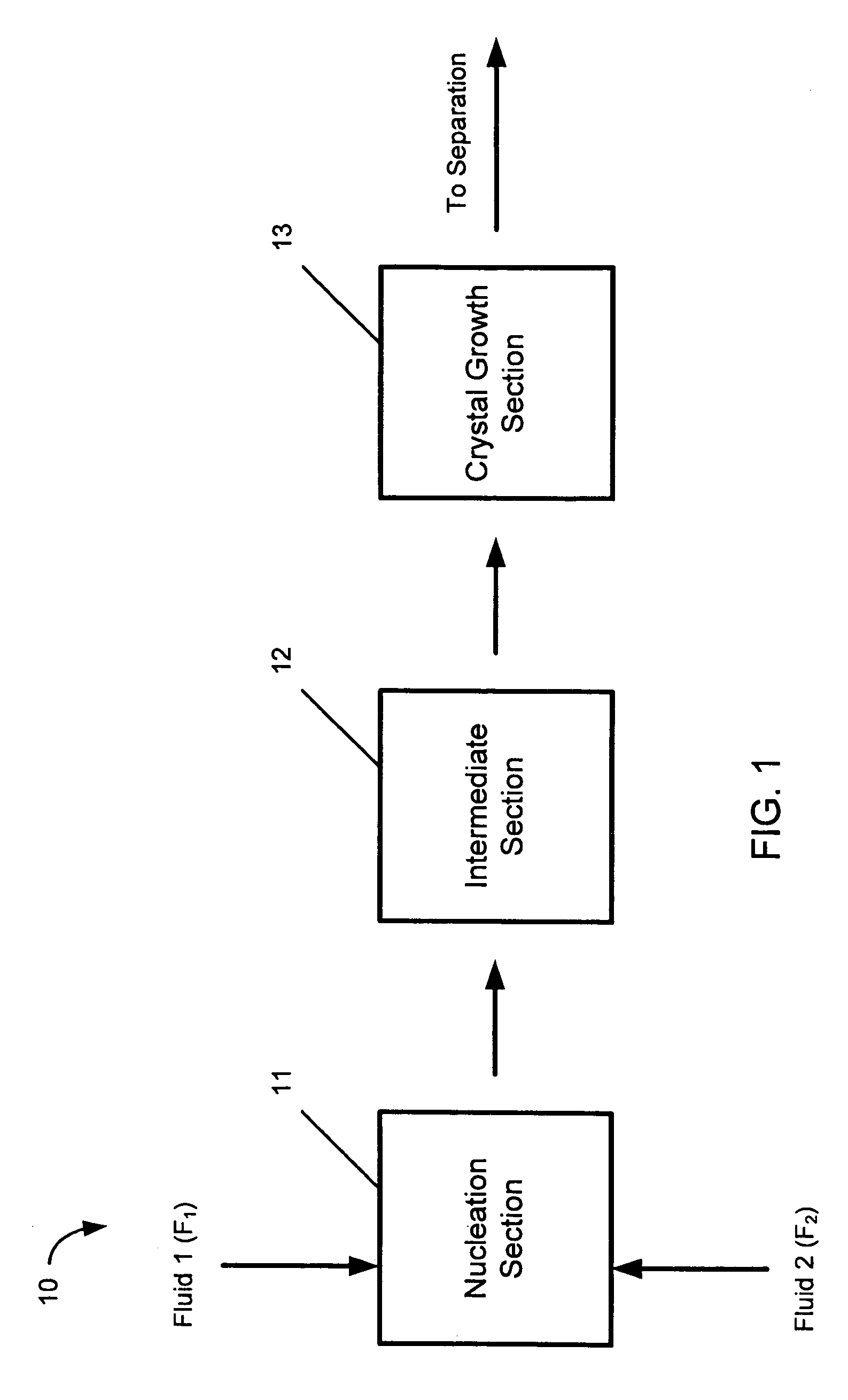
A device and process for crystallizing a compound using hydrodynamic cavitation comprising the steps of mixing at least one stream of a feed solution of such compound to be crystallized with at least one stream of an anti-solvent in a nucleating section via collision of the feed solution and the anti-solvent, passing the mixed streams at an elevated pressure through at least one local constriction of flow to create hydrodynamic cavitation thereby causing nucleation and the production of seed crystals, passing the fluid stream containing the seed crystals through an intermediate section to a crystal growth section, passing the fluid stream containing the seed crystals through the crystal growth section at an elevated pressure through at least one local constriction of flow to create hydrodynamic cavitation thereby causing further crystallization of the compound contained in the solution.
Owner:ILLINOIS INSTITUTE OF TECHNOLOGY +1
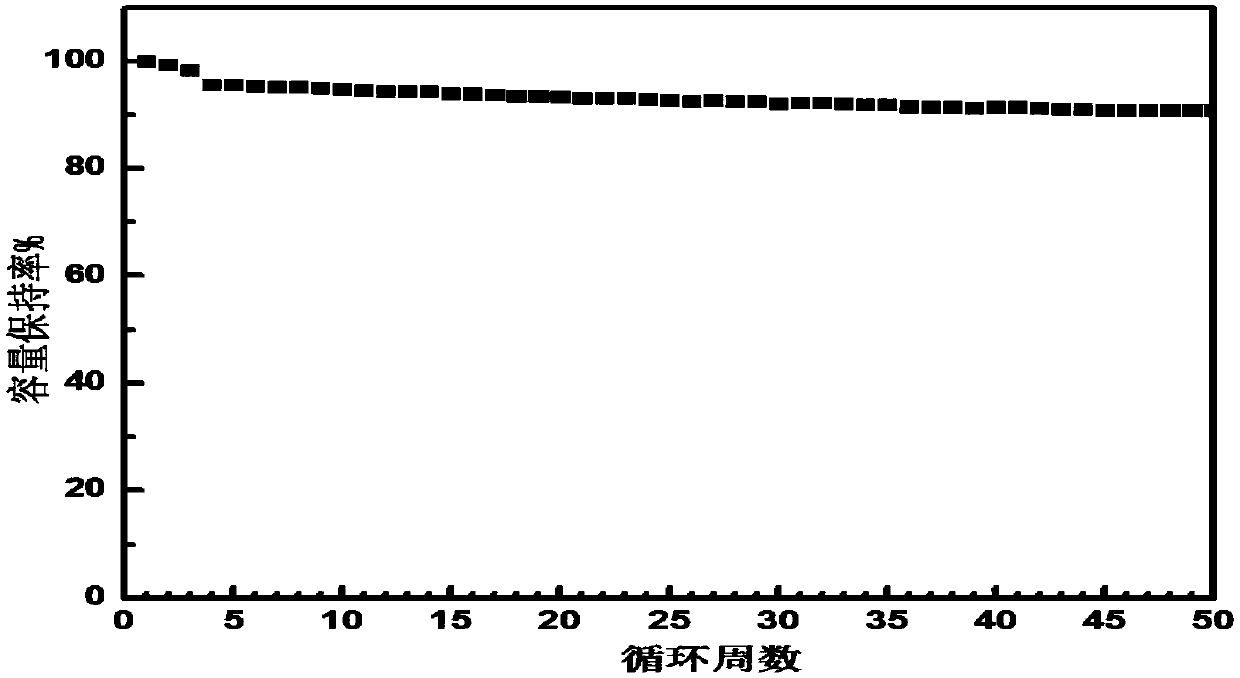
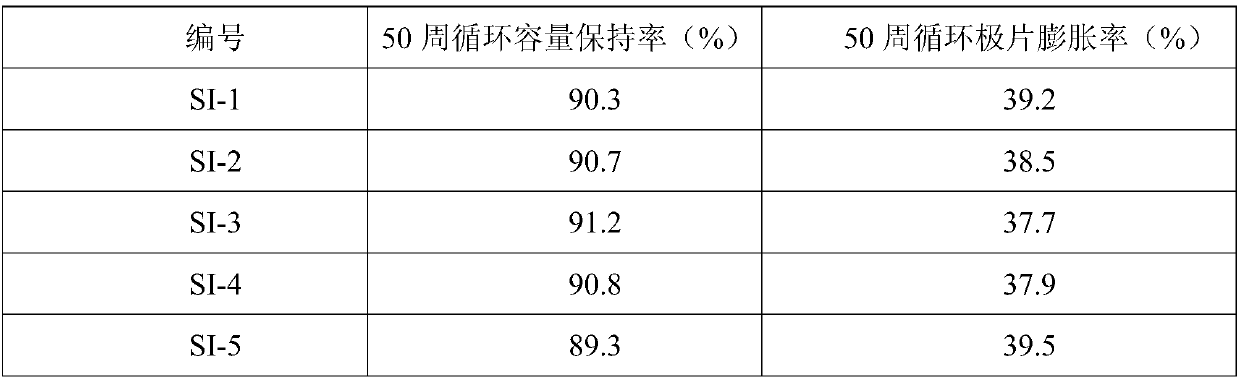
Silicon-based negative electrode material, preparation method thereof and application of silicon-based negative electrode material in lithium-ion battery
ActiveCN108054368AInhibit swellingImprove conductivityMaterial nanotechnologyElectrode thermal treatmentPolymer dissolutionAnti solvent
The invention relates to a silicon-based negative electrode material, a preparation method thereof and an application of the silicon-based negative electrode material in a lithium-ion battery. The silicon-based negative electrode material comprises a silicon-based active material and a composite layer, wherein the composite layer coats the surface of the silicon-based active material and is formedby a flexible polymer, flake graphite and a conductive material; and the method comprises the steps of (1) dissolving the flexible polymer into a solvent; (2) adding the flake graphite and the conductive material into a flexible polymer solution obtained in the step (1) under the stirring condition; (3) adding an anti-solvent to a mixed coating solution obtained in the step (2) and stirring; (4)adding the silicon-based active material to the supersaturated mixed coating solution obtained in step (3) under the stirring condition, stirring and separating; and (5) carrying out thermal treatmentto obtain the silicon-based negative electrode material. The silicon-based negative electrode material is simple in preparation method and low in cost, and industrial production is easy to implement;the prepared silicon-based negative electrode material has excellent electrochemical cycle performance and swelling inhibition performance, and the service life of the lithium-ion battery can be prolonged.
Owner:HUI ZHOU BTR NEW MATERIAL TECH
Polydopamine interface regulation-based nitramine explosive modified aluminum powder and preparation method thereof
ActiveCN109704896AEvenly distributedCompact structureExplosive working-up apparatusHigh energyAdhesive
The invention relates to a polydopamine interface regulation-based nitramine explosive modified aluminum powder and a preparation method thereof. Micron-sized crystals containing an aluminum powder and a nitramine explosive are prepared by an anti-solvent process, uniform distribution and close contact of the aluminum powder and the nitramine explosive are achieved, and the crystals have the advantages of compact structure, high thermal stability and high density. The adoption of the polydopamine interface regulation-based nitramine explosive modified aluminum powder in an aluminum-containingexplosive reduces the content of an adhesive and additives in a formula, increases the mass fraction of the main explosive, and achieves the purpose of developing high-energy explosives. In addition,a part of the energetic material adopted to replace RDX, HMX, CL-20 and other nitramine explosives in traditional solid propellants improves the energy level of the propellants, improves the problem of too large viscosity of a propellant slurry, caused by the addition of nano-aluminum powder or high-aluminum powder, and optimizes the preparation technology of the propellants.
Owner:NORTHWESTERN POLYTECHNICAL UNIV +1
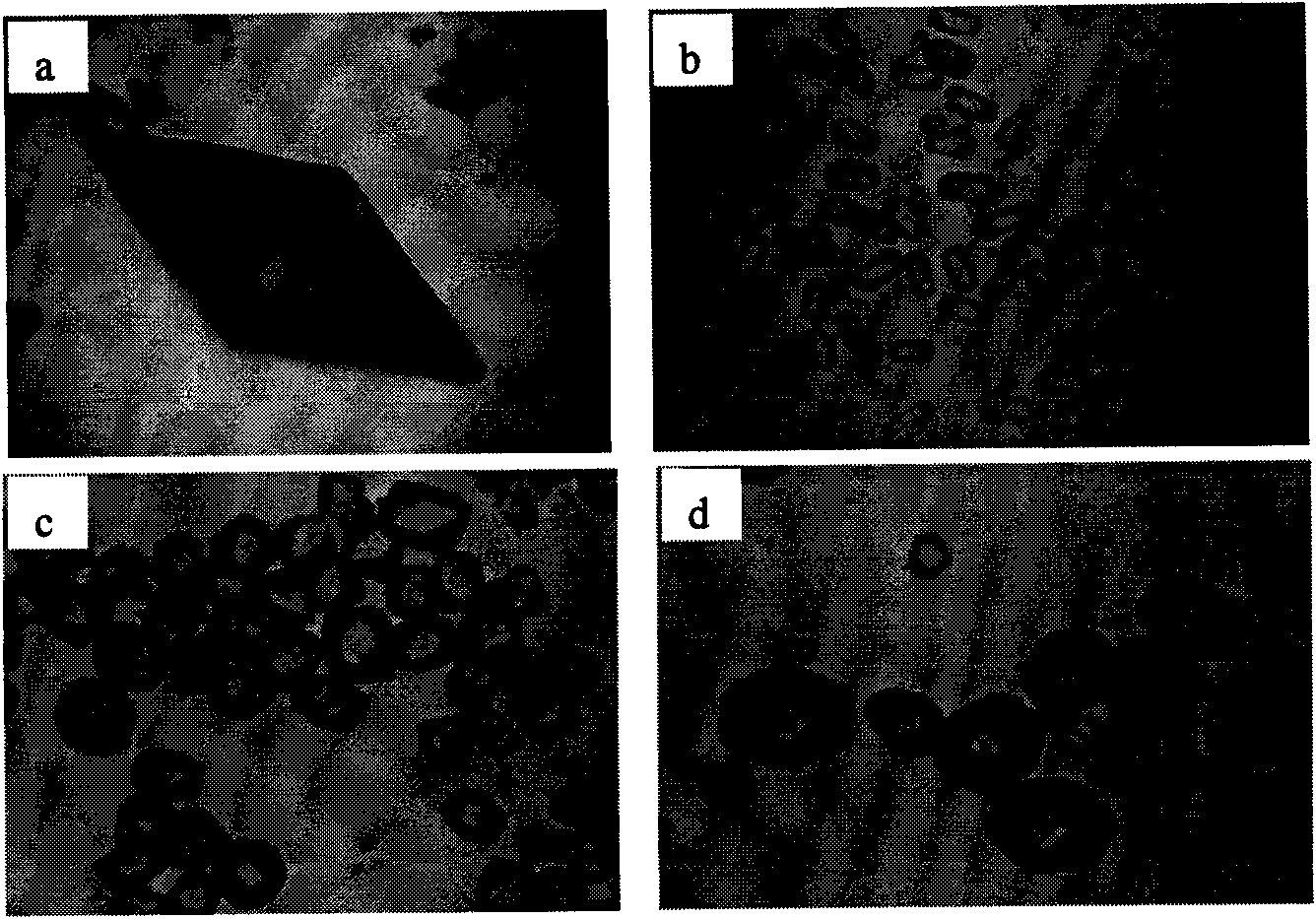
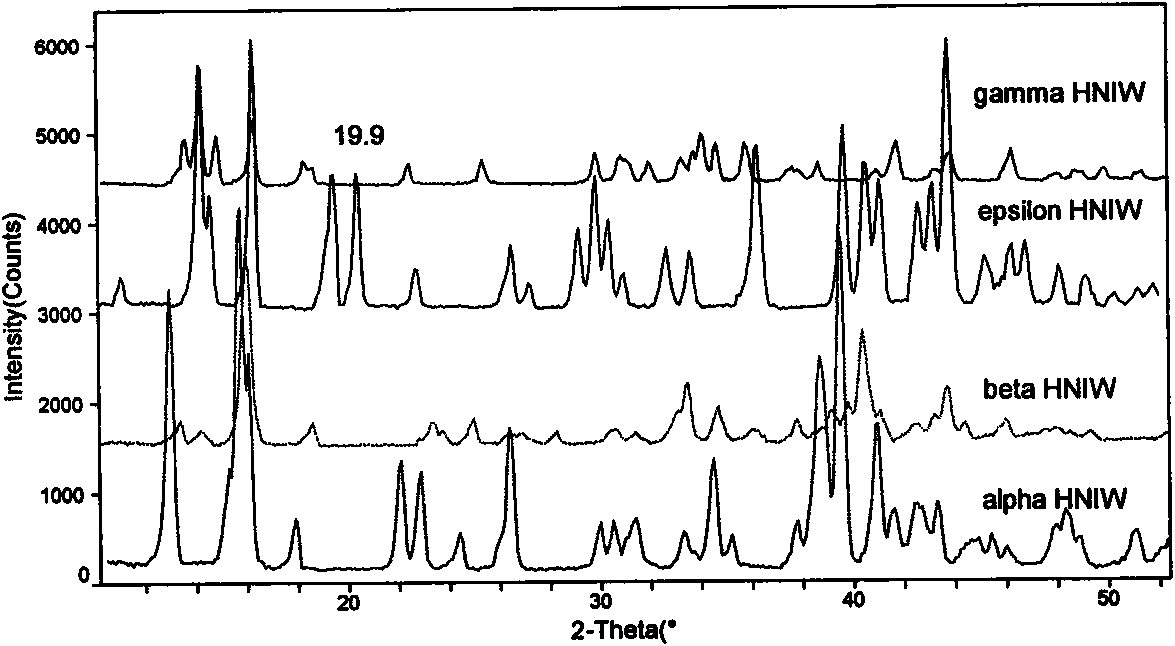
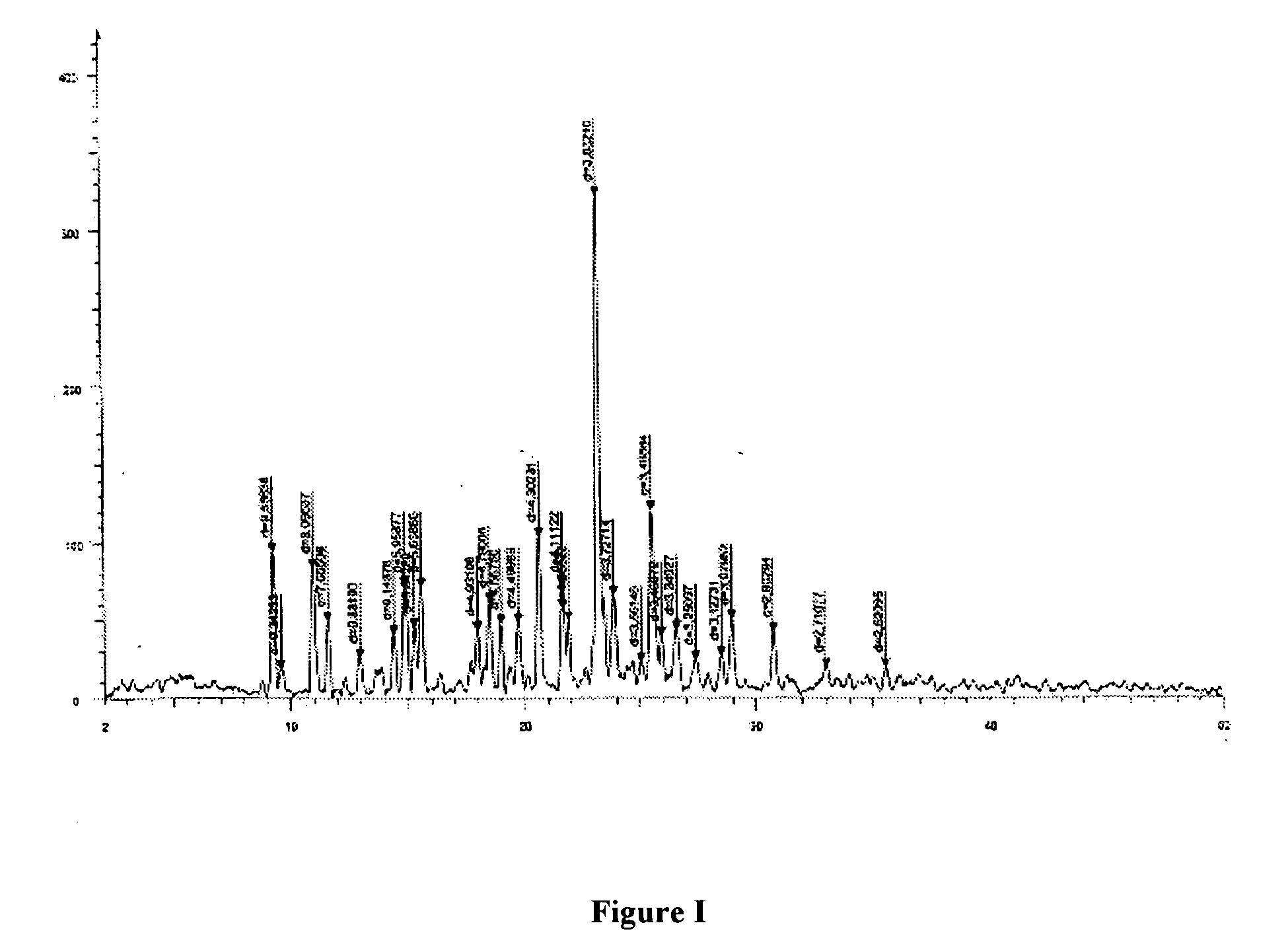
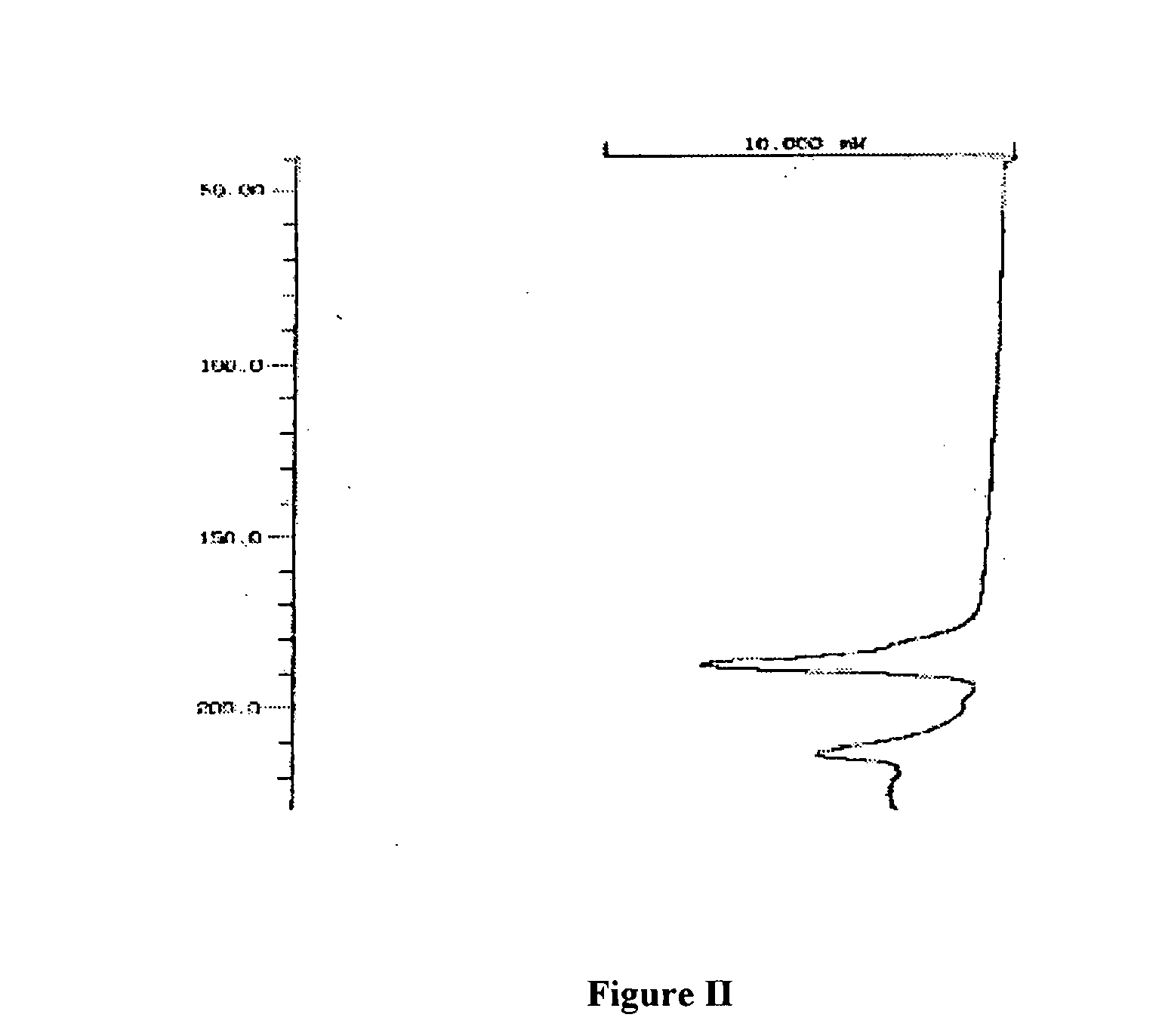
Sphericized hexanitrohexaazaisowurtzitane (HNIW) crystal and preparation method thereof
The invention provides a sphericized epsilon type hexanitrohexaazaisowurtzitane (HNIW) crystal and a preparation method thereof. The preparation method of the sphericized epsilon type hexanitrohexaazaisowurtzitane (HNIW) crystal comprises the following steps: a) dissolving alpha-HNIW, beta-HNIW and gamma-HNIW or mixture of the alpha-HNIW, the beta-HNIW and the gamma-HNIW into a solvent to form a saturated solution of the alpha-HNIW, the beta-HNIW and the gamma-HNIW or the mixture of the alpha-HNIW, the beta-HNIW and the gamma-HNIW; b) adding a crystal growth control agent to the saturated solution and adding an anti-solvent to form a supersaturated solution; and c) adding epsilon-HNIW seed crystals to the supersaturated solution and continuously adding the anti-solvent until all HNIW in the supersaturated solution is released to grow a sphericized epsilon-HNIW crystal. The sphericized epsilon-HNIW crystal does not have sharp edges and corners but has a multi-faceted spherical shape, particle size of 20 to 500 um, chemical purity of 98 to 99.9 percent determined by high performance liquid chromatography, porosity of 0.01 percent to 0.40 percent and 5kg drop hammer impact sensitivity H50 of 26 to 52 centimeters.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Novel process for preparation of clopidogrel bisulfate polymorph - Form I
A process for making Clopidogrel Bisulfate Form I which comprises dissolving Clopidogrel Bisulfate Form II in a solublizing solvent at room temperature to form a solution; adding an anti-solvent to the said solution till turbid; stirring the said turbid solution; collecting the precipitated solid and drying the final solid product, form I.
Owner:SAWANT KAMLESH DIGAMBAR +2
Preparation method of ezetimibe tablet
The invention discloses a preparation method of an ezetimibe tablet. The preparation method comprises the following steps of (1) dissolving hydroxypropyl cellulose and ezetimibe into ethanol, and dissolving a carrier material mannitol into a water solution; (2) respectively introducing a supercritical carbon dioxide fluid and the two solutions into a coaxial three-channel nozzle by setting the pressure and temperature of the supercritical carbon dioxide fluid to 10-50 MPa and 35-60 DEG C, and acquiring ultrafine granule mixed powder which contains the ezetimibe, the hydroxypropyl cellulose and the mannitol by utilizing the anti-solvent effect of the supercritical carbon dioxide fluid; (3) directly tabletting the ultrafine granule mixed powder and pharmaceutically acceptable auxiliary materials. The method disclosed by the invention can be used for fast dissolving the ezetimibe, can achieve the dissolution rate of the ezetimibe tablet by 95% for 5 minutes and does not contain the surface active agent.
Owner:QINGDAO UNIV OF SCI & TECH
Method for preparing sustained-release miniball
The invention provides a method for preparing sustained-release miniball, which comprises dissolving polypeptides or micromolecular proteins into DMSO, charging the solution into DCM containing PLGA, forming fine particles of the medicament by utilizing anti-solvent, charging the formed suspension into outer aqueous phase, finally volatilizing the organic solvent to obtain slow release microballoons.
Owner:SHANGHAI HUAYI BIO LAB CO LTD
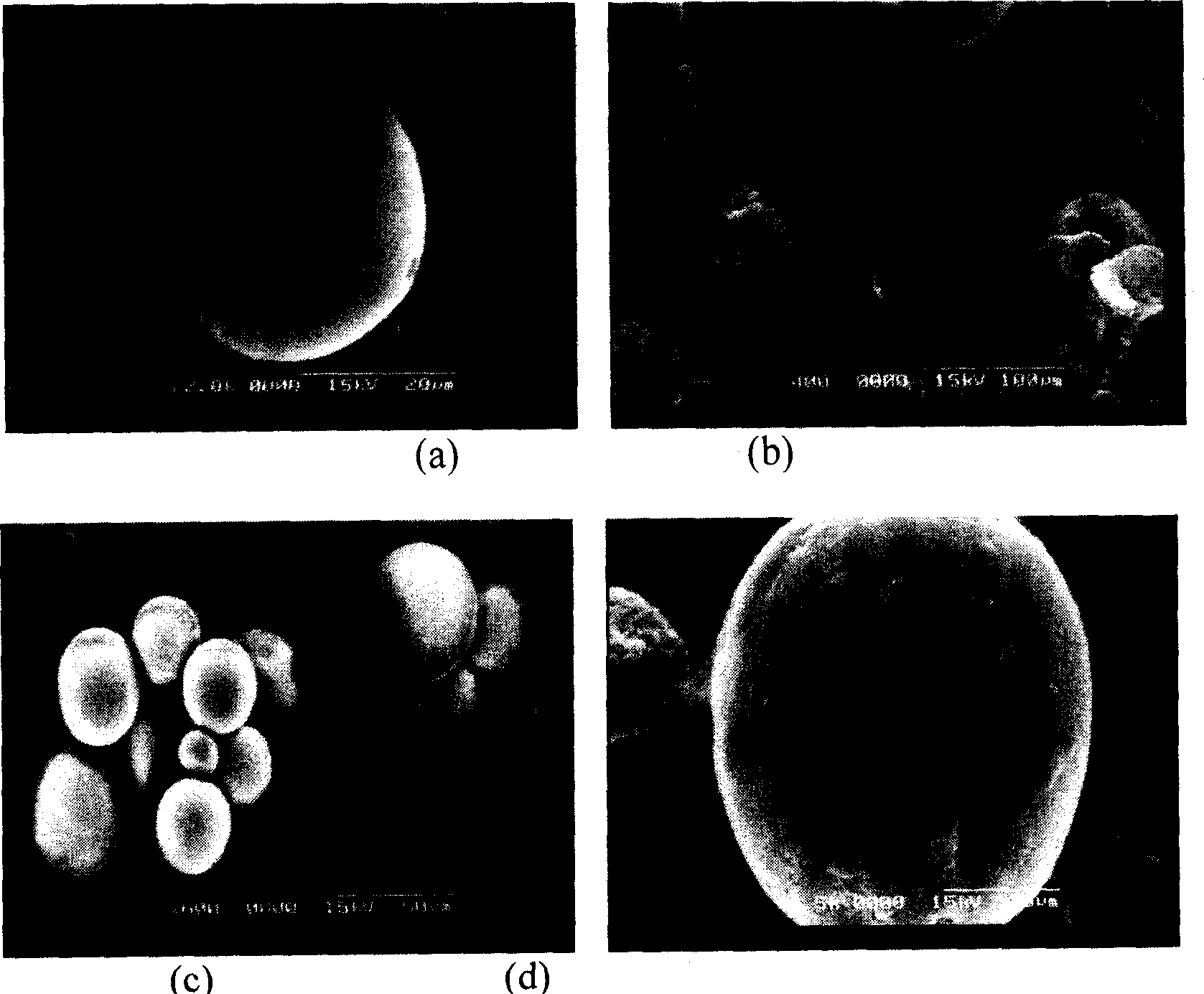
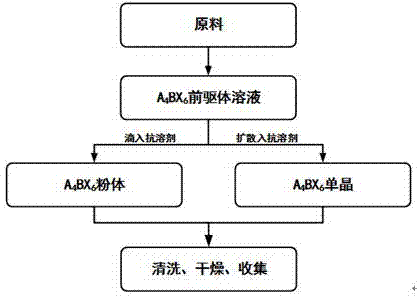
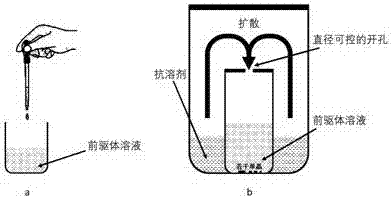
Zero dimension perovskite structure luminescent material A4BX6 and preparation method thereof
ActiveCN107267144AImprove luminous efficiencySingle crystal with few defectsPolycrystalline material growthTin compoundsSolubilityAnti solvent
The invention discloses a zero dimension perovskite structure luminescent material A4BX6 and a preparation method thereof. The preparation method comprises the steps of utilizing a principle that the solubility of a solute in a precursor solution can be reduced through an anti-solvent, dripping or slowly diffusing the anti-solvent into the precursor solution so as to prepare the zero dimension perovskite structure efficient luminescent material A4BX6 powder or a single crystal, and concretely, dissolving an A-containing halogen compound and a B-containing halogen compound or B-containing oxide into a solvent to obtain the precursor solution; slowly dripping or diffusing the precursor solution into the anti-solvent, and separating out the zero dimension perovskite structure luminescent material A4BX6 from the precursor solution. The method has the advantages that the obtained zero dimension perovskite structure luminescent material A4BX6 has high purity, the luminous efficiency is high, the requirement on the used equipment is simple, and the like.
Owner:KUNMING UNIV OF SCI & TECH
Low-temperature fast-curing epoxide powder paint and preparation method thereof
ActiveCN103666203AImprove water resistanceGood solvent resistanceAnti-corrosive paintsEpoxy resin coatingsPolymer scienceFirming agent
The invention relates to low-temperature fast-curing epoxide powder paint which comprises the following components in percentage by weight: 45-65% of epoxy resin, 1-5% of polyepoxy active crosslinking agent, 8-18% of curing agent, 0.1-0.5% of curing accelerating agent, 4-30% of precipitated barium sulfate, 4-8% of mica powder, 2-5% of titanium white, 0.5-1.0% of adhesion accelerating agent and assistants. According to the invention, the epoxide powder paint uses a blended resin system of high molecular weight bisphenol A type epoxy resin and novolac epoxy resin; and after being cured by the curing agent, an obtained paint film has the advantages of the epoxy resin such as high adhesion and good alkali resistance and the characteristics of the novolac resin such as favorable water resistance, solvent resistance and acid resistance, and the paint film has favorable heat resistance. Thus, the epoxide powder paint provided by the invention can be adapted to a high-temperature severe-corrosion environment and has favorable market application prospects.
Owner:洛阳双瑞防腐工程技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com