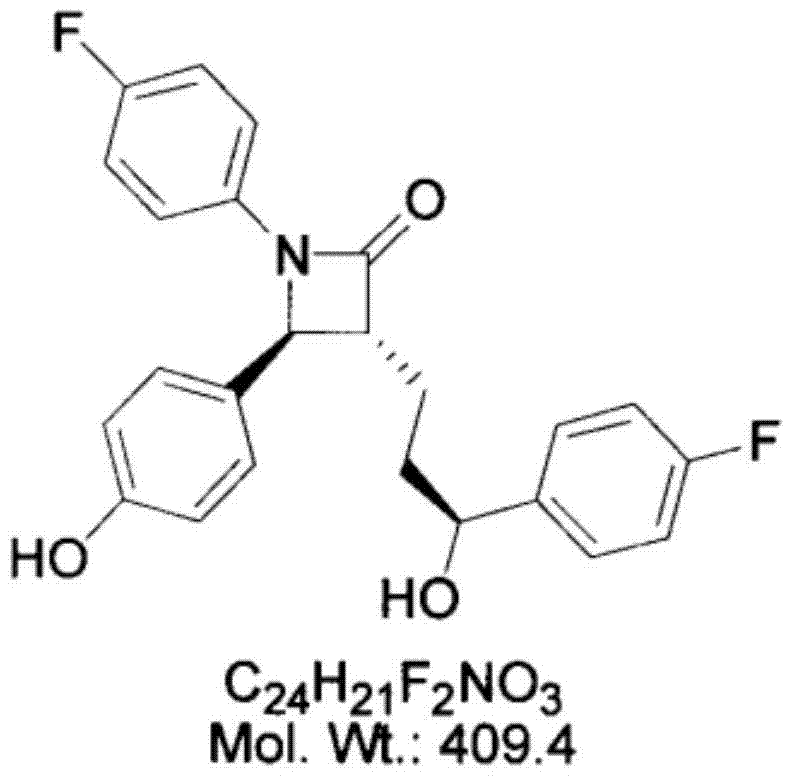
Preparation method of ezetimibe tablet
A technology of ezetimibe and hydroxypropyl cellulose, which is applied to medical preparations containing no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. Problems such as the production process of wheat cloth flakes and the inability to fully demonstrate the superiority of micronization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] Preparation Process:
[0029] (1) Hydroxypropyl cellulose and ezetimibe were weighed for the prescription amount, dissolved in ethanol, and mannitol was weighed for the prescription amount and dissolved in water;
[0030] (2) Set the pressure of the supercritical carbon dioxide fluid to 10MPa and the temperature to 35°C, pass the supercritical carbon dioxide fluid and the above two solutions into a coaxial three-channel nozzle respectively, and use the anti-solvent effect of the supercritical carbon dioxide fluid to obtain Superfine powder blend of ezetimibe, hydroxypropyl cellulose and mannitol;
[0031] (3) Mix the superfine particle mixed powder with microcrystalline cellulose, crospovidone and magnesium stearate evenly, and directly compress it into tablets.
Embodiment 2
[0033]
[0034]
[0035] Preparation Process:
[0036] (1) Hydroxypropyl cellulose and ezetimibe were weighed for the prescription amount, dissolved in ethanol, and mannitol was weighed for the prescription amount and dissolved in water;
[0037] (2) Set the pressure of the supercritical carbon dioxide fluid to 50MPa and the temperature to 60°C, pass the supercritical carbon dioxide fluid and the above two solutions into a coaxial three-channel nozzle respectively, and use the anti-solvent effect of the supercritical carbon dioxide fluid to obtain Superfine powder blend of ezetimibe, hydroxypropyl cellulose and mannitol;
[0038] (3) Mix ultrafine granule mixed powder with microcrystalline cellulose, starch, crospovidone, sodium carboxymethyl starch, and zinc stearate, and directly compress into tablets.
Embodiment 3
[0040]
[0041] Preparation Process:
[0042] (1) Hydroxypropyl cellulose and ezetimibe were weighed for the prescription amount, dissolved in ethanol, and mannitol was weighed for the prescription amount and dissolved in water;
[0043] (2) Set the pressure of the supercritical carbon dioxide fluid to 30MPa and the temperature to 50°C, pass the supercritical carbon dioxide fluid and the above two solutions into a coaxial three-channel nozzle respectively, and use the anti-solvent effect of the supercritical carbon dioxide fluid to obtain Superfine powder blend of ezetimibe, hydroxypropyl cellulose and mannitol;
[0044] (3) Mix the superfine particle mixed powder with microcrystalline cellulose, crospovidone and magnesium stearate evenly, and directly compress it into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com