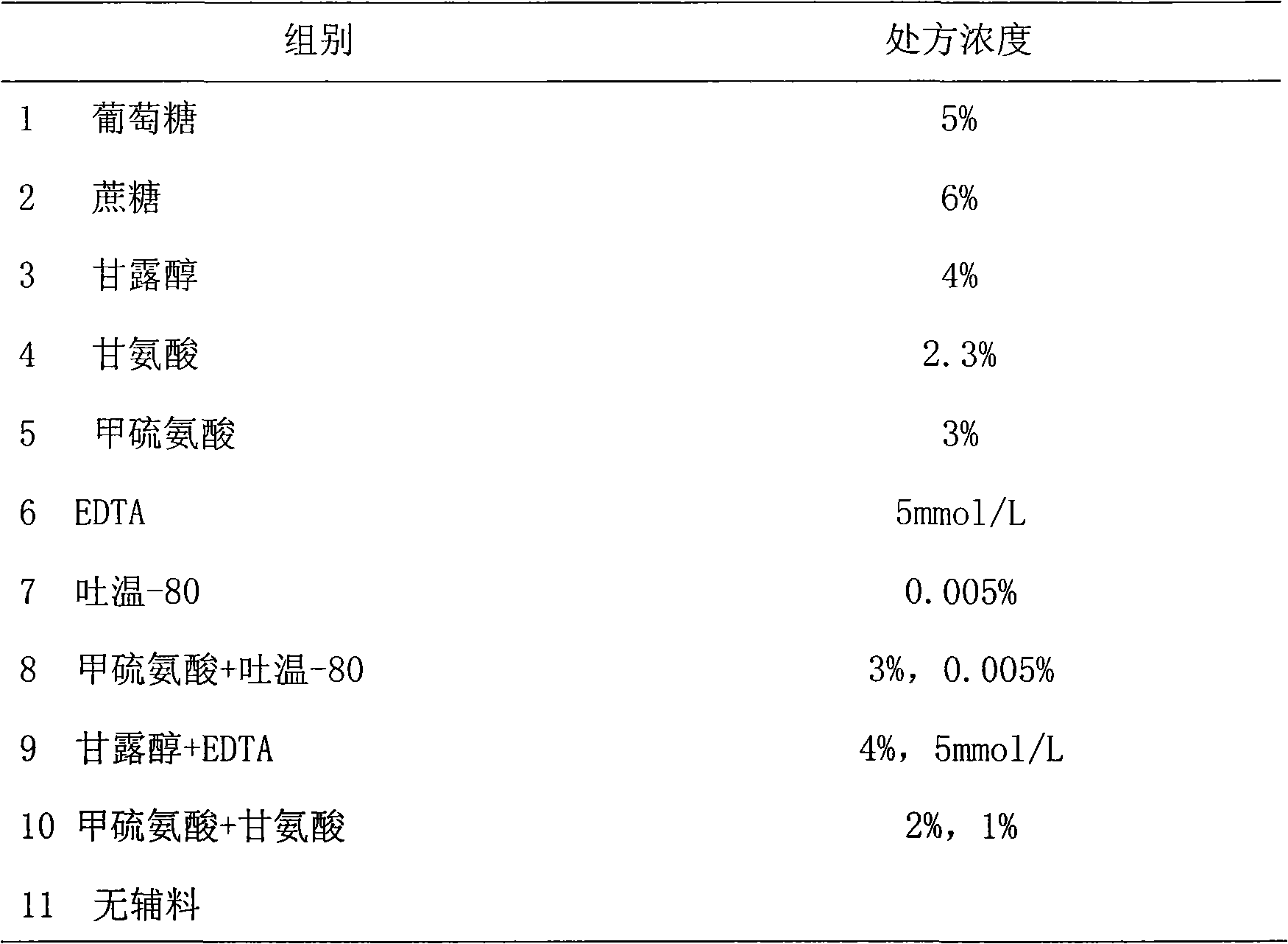
Medicinal preparation containing exenatide
A pharmaceutical preparation, exenatide technology, applied in the field of exenatide stable pharmaceutical preparations, can solve the problems of exenatide stability cannot be guaranteed, affect the curative effect, and dosage changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
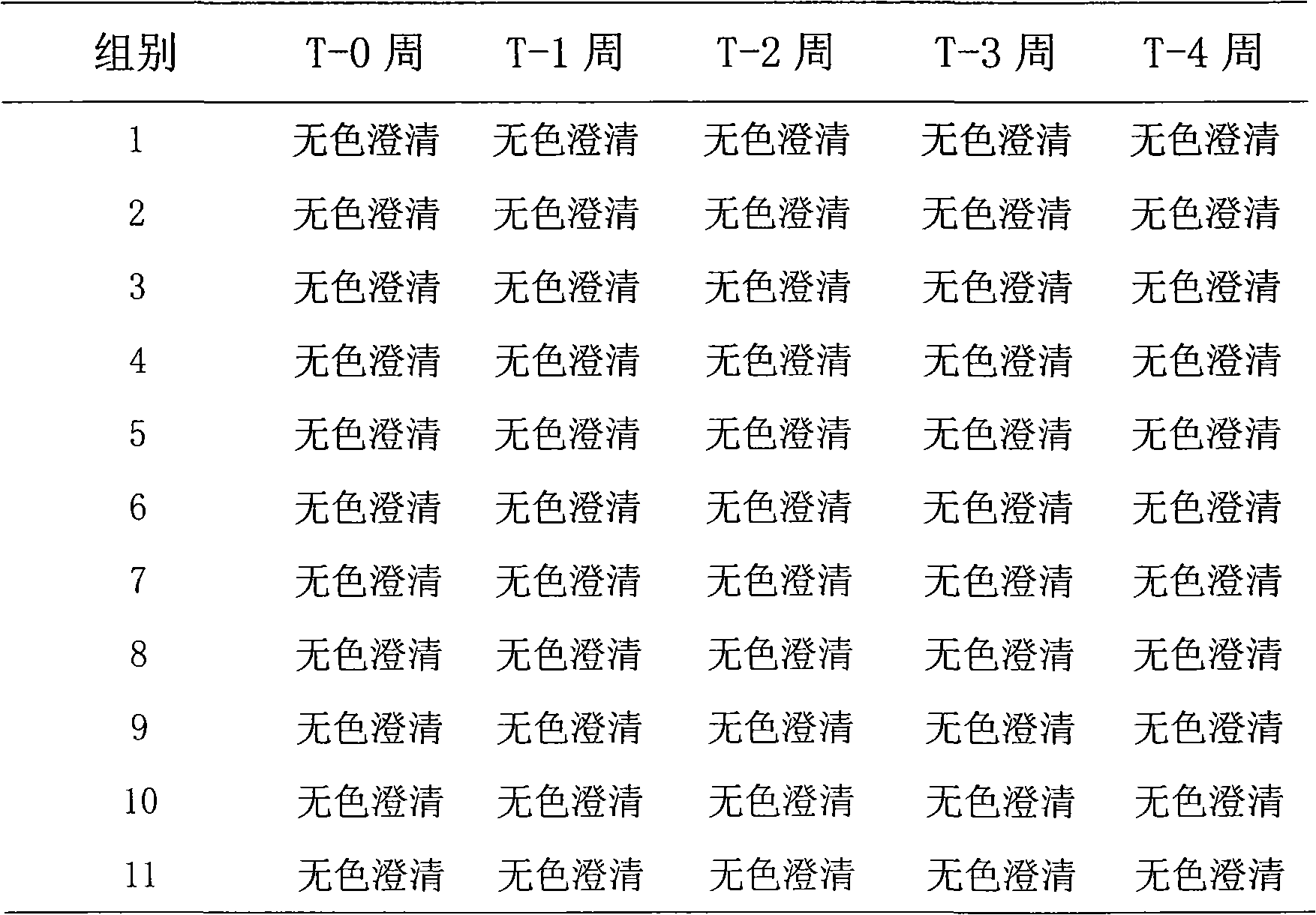
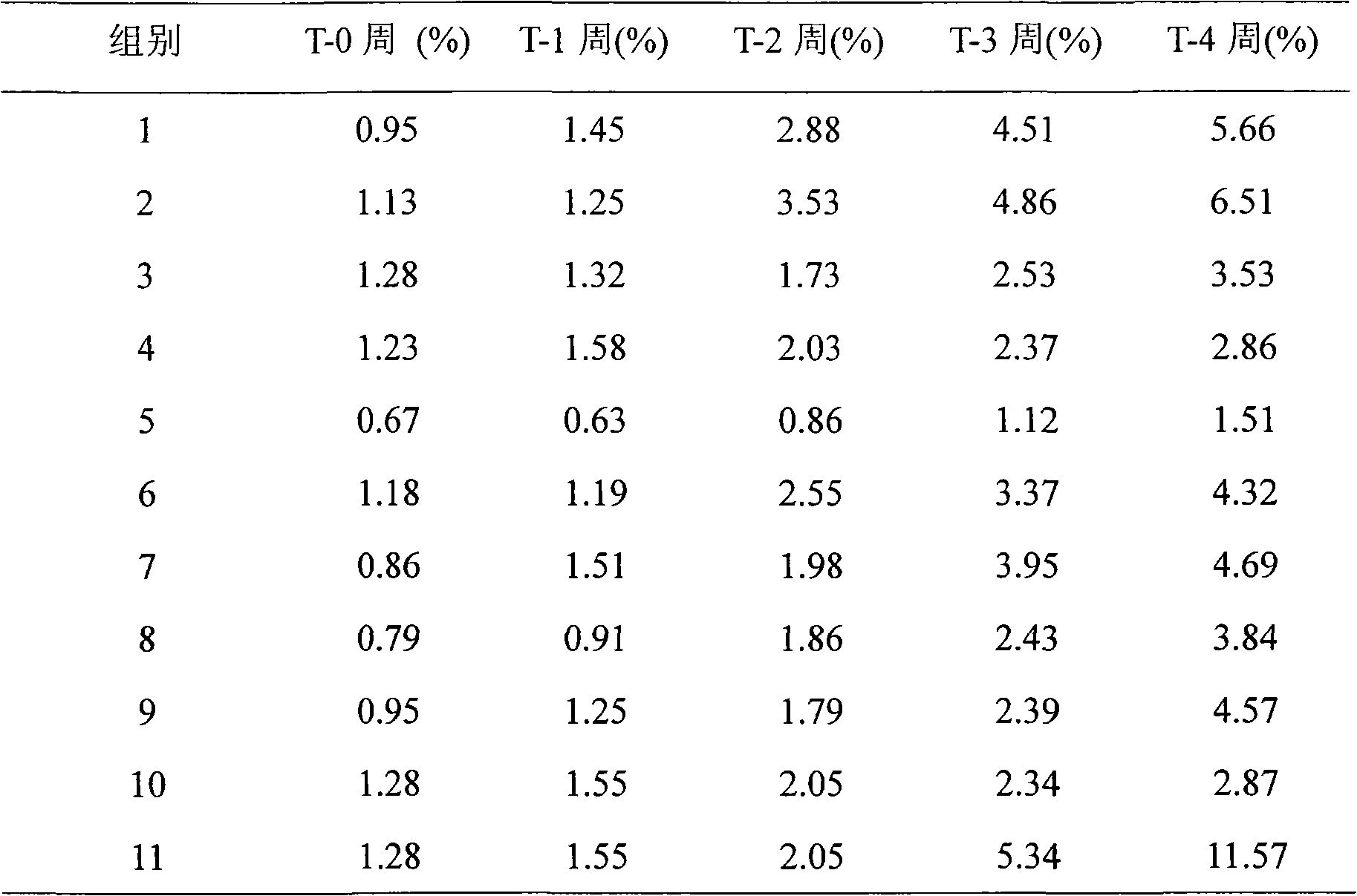
[0069] Example 1: Screening of preservatives
[0070] In order to study the stability of exenatide under the condition of adding different preservatives, the following experiment was carried out, the method is as follows: first prepare different preservative solutions, each solution contains 3% methionine and 20mM pH 4.5 Disodium hydrogen phosphate-citric acid buffer solution, then weigh exenatide, add it to different preservative solutions and dissolve it into an exenatide preparation solution of about 250 μg / ml, and then use NaOH or HCl to adjust the pH to 4.5. After the preparation, put it into a 25°C incubator for accelerated testing. Samples were taken every 2 weeks and tested by HPLC to observe the degradation of the samples. The specific results are shown in the table below.
[0071] Byetta prescription: Exenatide 250μg / ml + m-cresol 2.2mg / ml + 4% mannitol + 30mM acetate buffer
[0072] Table 6: Addition of different preservatives
[0073]
[0074] Table 7: Conten...
example 2
[0077] Example 2: Screening of different concentrations of sodium benzoate
[0078] First prepare sodium benzoate solutions containing different concentrations, each solution contains 3% methionine and 20mM pH 4.5 disodium hydrogen phosphate-citric acid buffer, then weigh exenatide, add it to different Dissolve in preservative solution to form about 250 μg / ml exenatide preparation solution, then adjust pH to 4.5 with NaOH or HCl. After the preparation, put it into a 25°C incubator for accelerated testing. Samples were taken every 2 weeks and tested by HPLC to observe the degradation of the samples. The specific results are shown in the table below.
[0079] Table 8: Periodic reverse-phase detection of the content of related substances
[0080]
[0081] Can get from above-mentioned result: the sodium benzoate of 0.005%-5% (especially 0.2%-2.0%) has antiseptic effect preferably, but considers that preservative generally has certain toxic effect to human body, therefore adopt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com