Additive solution for blood preservation
a technology of additive solutions and blood, which is applied in the field of additive solutions for blood preservation, can solve the problems of periodic shortages of blood, limited liquid blood supply, and patients currently cannot collect and store with current technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
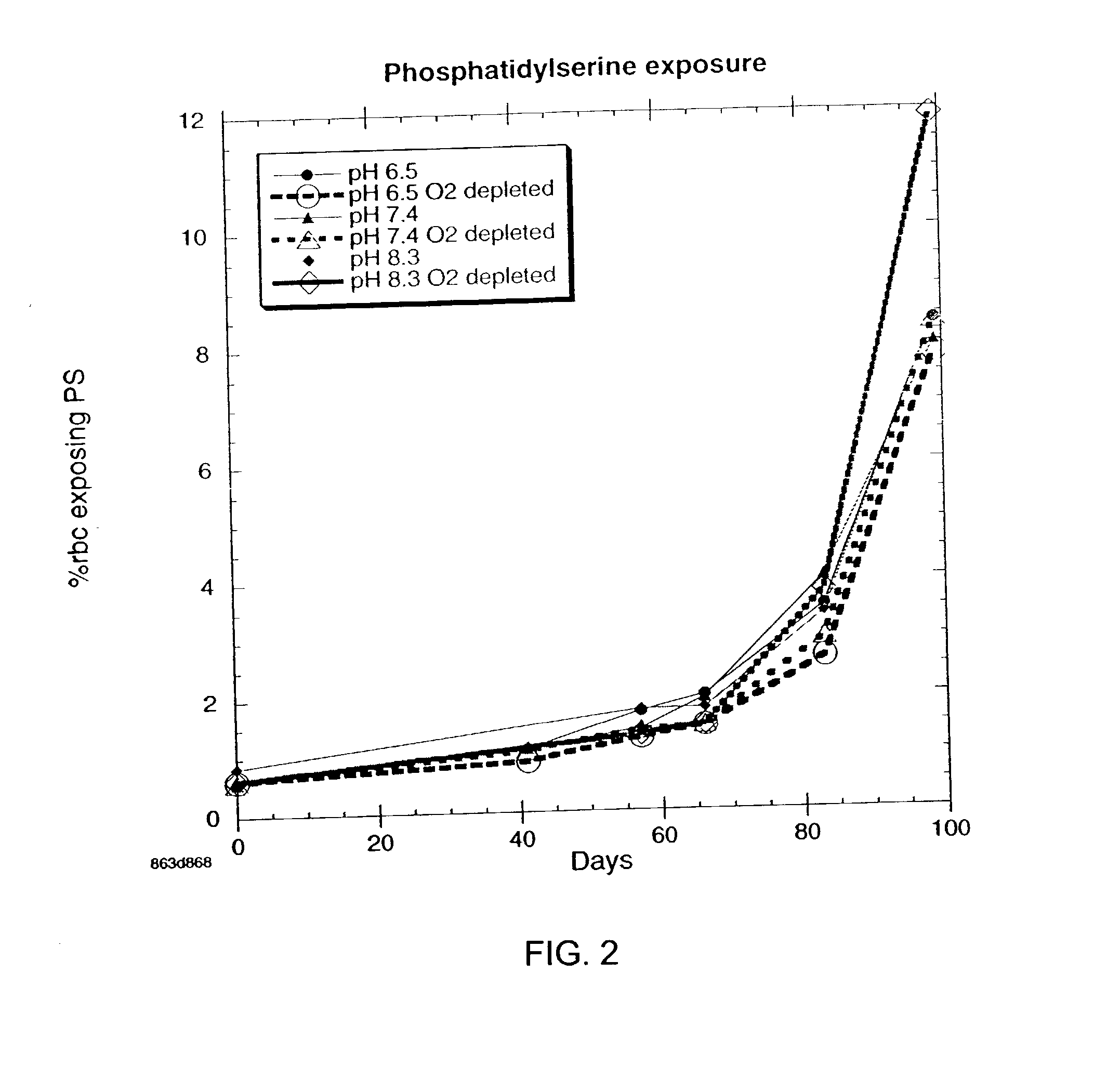
OFAS3: Effect of pH and Oxygen Depletion on % of Cells Exposing Phosphatidylserine
[0034] Results of experimentation to determine the effect of pH and oxygen depletion on the % of red blood cells exposing phosphotidylserine with samples containing oxygen-free additive solution (OFAS3) are presented in FIG. 2. Data were obtained by flow cytometer measurements using FITC-Annexin IV probe. Each point on the graph is the average of 6 subjects. There is a significant reduction in exposed phosophatidylseine after 10 weeks when pH 8.3 and pH 6.5 blood samples, both oxygen depleted, are compared.
example 3
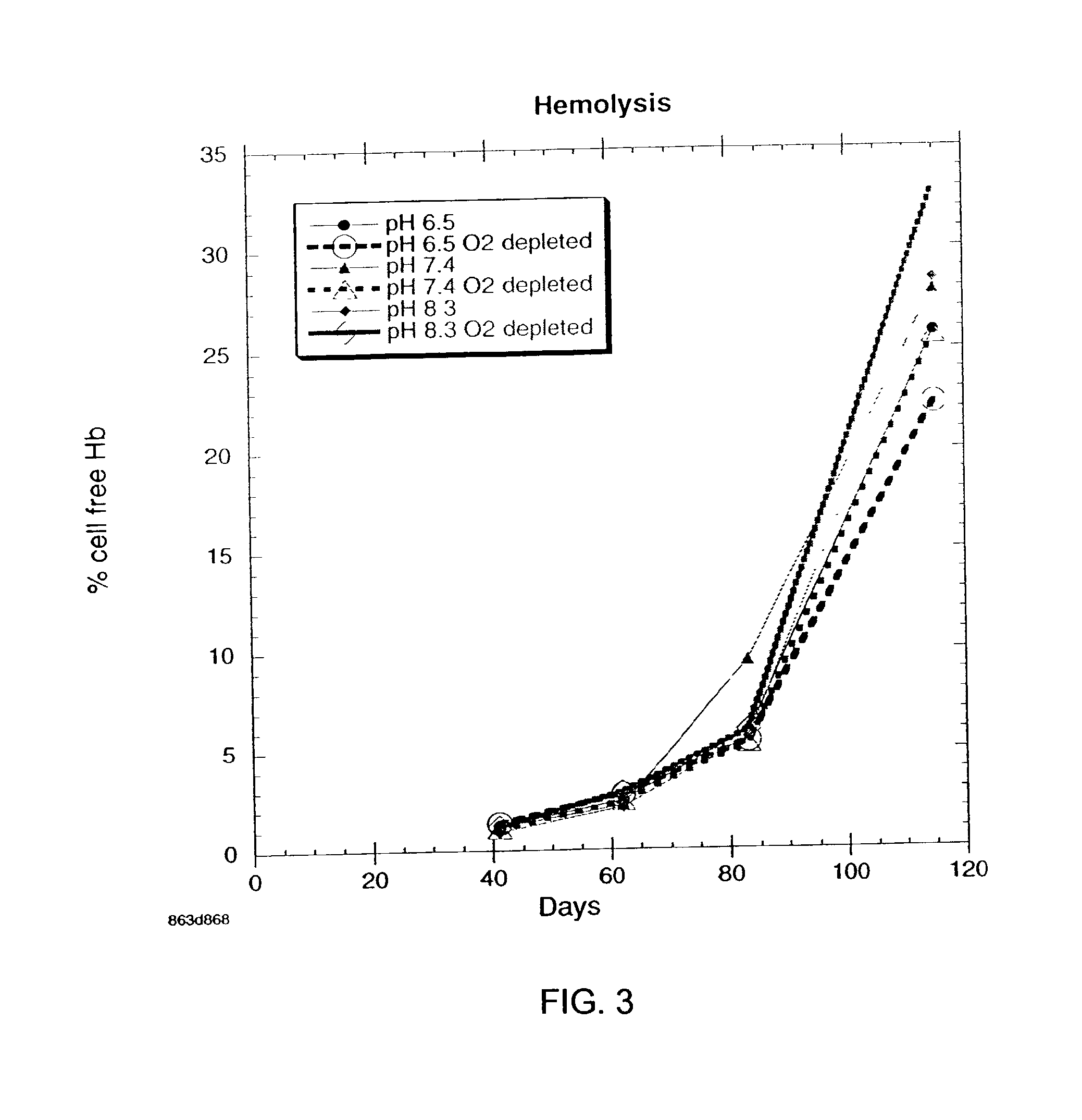
OFAS3: Effect of pH and Oxygen Depletion on Red Blood Cell Hemolysis
[0035] Results of experimentation to determine the effect of pH and oxygen depletion on red blood cell hemolysis with blood samples containing oxygen-free additive solution (OFAS3) are presented in FIG. 3. Each point on the graph is the average of 6 subjects. Three different pH's were tested, pH 6.5, pH 7.4, and pH 8.3, with control cultures that were not oxygen-depleted. At week 16, the pH 6.5 oxygen-depleted refrigerated red blood cell storage system has the lowest extent of hemolysis.
example 4
Addition of Metabolic Supplements During Refrigerated, Oxygen-Depleted Red Blood Cell Storage: Effect of Metabolic Supplements Added at Different pH's in the Presence or Absence of Oxygen on Cellular ATP Levels
[0036] Results of experimentation to determine the effect of addition of metabolic supplements added during refrigerated, oxygen-depleted storage of red blood cells at different pH's in the presence or absence of oxygen on cellular ATP levels, are graphically presented in FIG. 4. Two different pH's were tested, pH 6.5 and pH 8.3, with control cultures that are not oxygen depleted. Metabolic supplement, Rejuvesol, was added to cultures as indicated by the arrows in FIG. 4, which correspond approximately to additions during cold storage at 9, 14, and 21 weeks respectively. These data show that ATP levels are significantly increased each time the cold fuel / metabolic supplement is added. The highest ATP levels are sustained with pH 6.5 additive solution under oxygen depleted condi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com