Method for preparing sustained-release miniball
A technology of slow-release microspheres and active ingredients, applied in the direction of powder transportation, etc., can solve the problems of large burst release, insufficient release volume, unsatisfactory slow-release results, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Typical protein, polypeptide powder and protein powder particle size comparison obtained by anti-solvent method
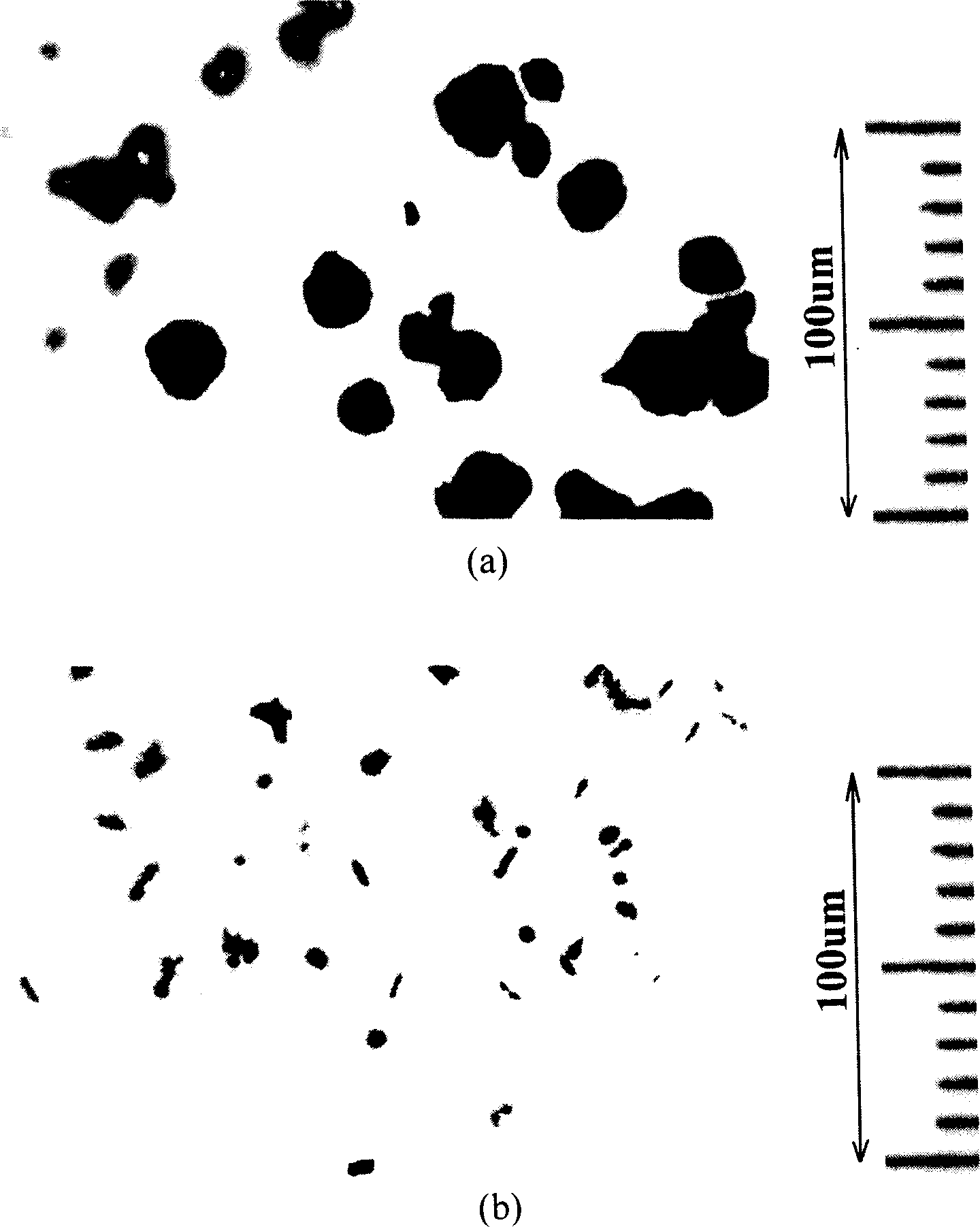
[0055] 1) Take a 1ml eppendorf centrifuge tube, add 1ml of alcohol, then add 5mg of porcine insulin freeze-dried powder (purchased from Jiangsu Wanbang Biochemical Co., Ltd.), mix by ultrasonic for 15s, then take 1 drop of the suspension and drop it on a glass slide, place it under a microscope observe. picture
[0056] 2(a) is the observed image.
[0057]2) The above lyophilized porcine insulin powder was dissolved in DMSO at a concentration of 50 mg / ml. Take 100 μl of the solution and add it to 900 μl of ethanol, vortex quickly to mix, then take 1 drop of the suspension and drop it on a glass slide, and place it under a microscope for observation. figure 2 (b) is the observed image.
[0058] From figure 2 It can be seen from the results that the average particle size of insulin freeze-dried powder is about 20-30 μm, and the anti-solvent m...
Embodiment 2
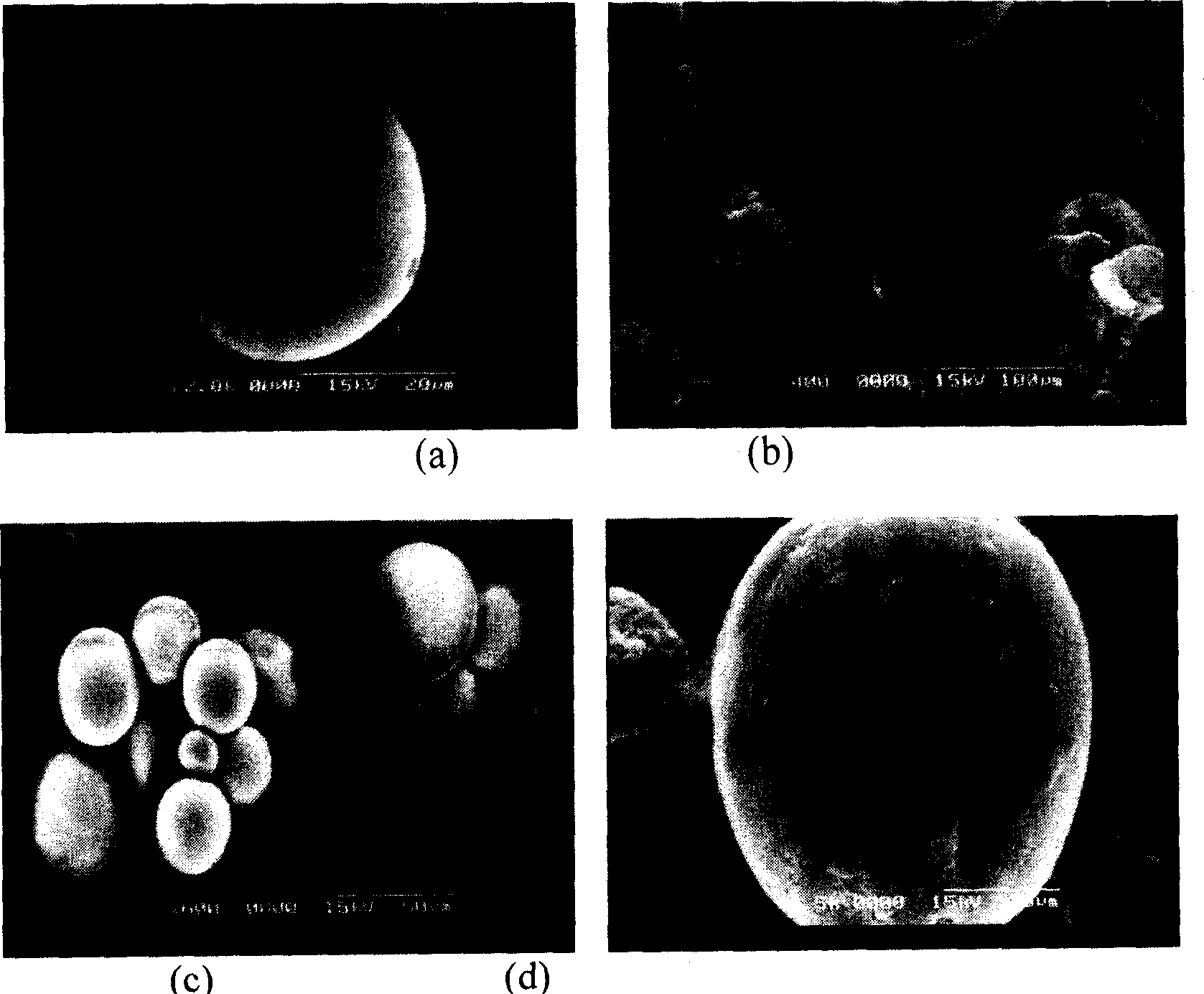
[0060] Comparison of preparation of microspheres by traditional W1 / O / W2 double emulsification method, traditional S / O / W double emulsification method and improved S / O / W method
[0061] The polymer used in this example is polylactide-co-glycolide (polylactide-co-glycolide), wherein polylactide / glycolide (lactide / glycolide) = 50 / 50, namely PLGA (50:50) , with an intrinsic viscosity (ie IV) of 0.39, purchased from Birmingham Polymers, USA.
[0062] 1) W1 / O / W2 method: prepare 25mg / ml and 50mg / ml porcine insulin aqueous solutions, respectively take a certain volume of solution and add 2ml of dichloromethane (DCM) dissolved with 100mgPLGA (50:50), use high-speed homogenizer Homogenize for 1.5 minutes with a machine (F6 / 10 model, Fluko, Germany) at 10,000-15,000 rpm. Then, the W1 / O primary emulsion was added into 200 ml of 1.0% (W / V) polyvinyl alcohol (PVA, sigma, USA) solution, and stirred by a magnetic stirrer for 3 hours (1000 rpm). Afterwards, the microspheres were collected by ...
Embodiment 3
[0076] Embodiment 3: Encapsulate GLP-1(7-36)OH with improved S / O / W method
[0077] According to the method shown in Experiment 3) in Example 2, PLGA with different internal viscosities (molecular weights) were selected to prepare microspheres encapsulated with GLP-1(7-36)OH. The results are shown in Table 3.
[0078] Numbering
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com