Patents
Literature
30 results about "Oxazolidinedione" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
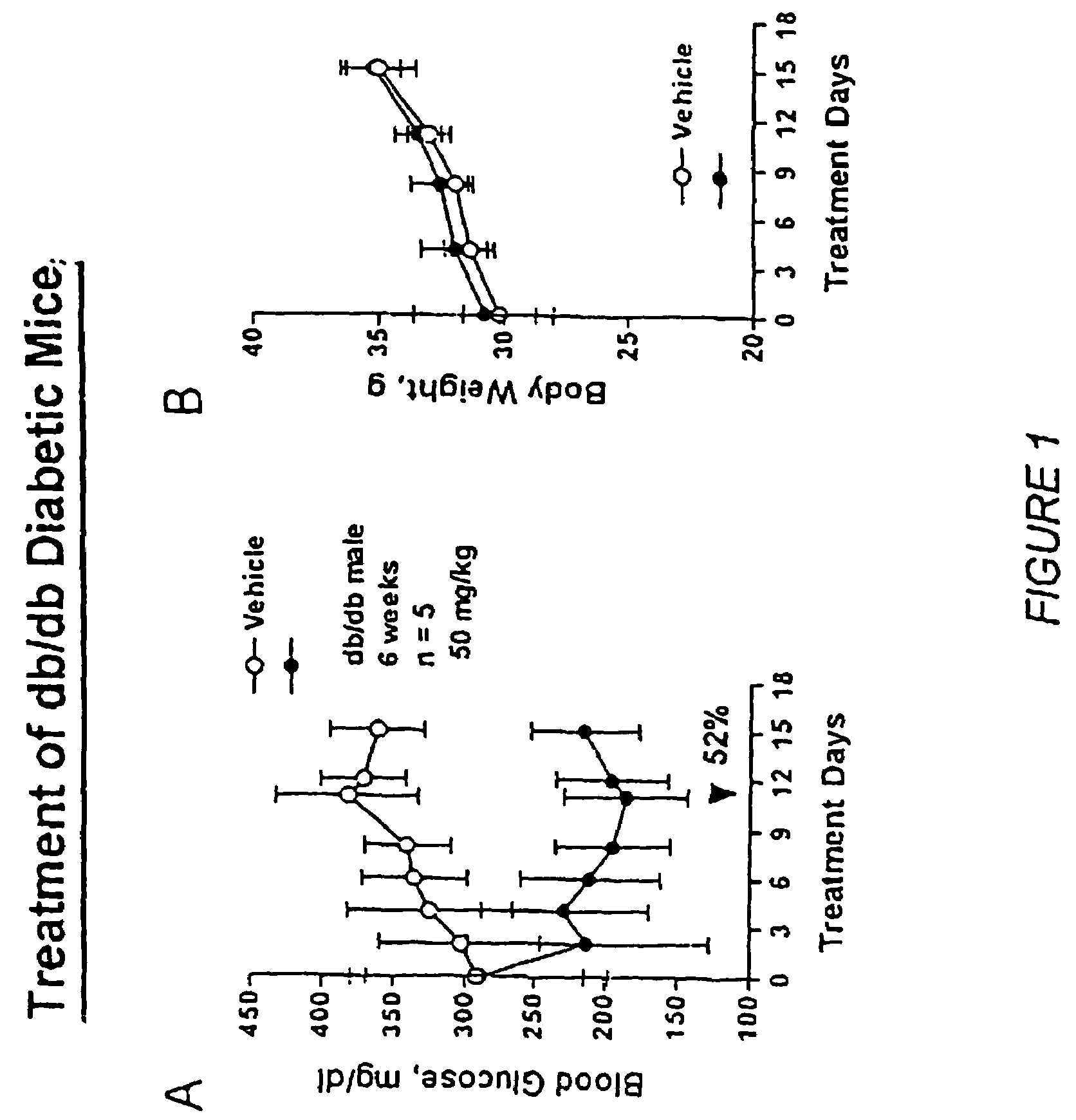
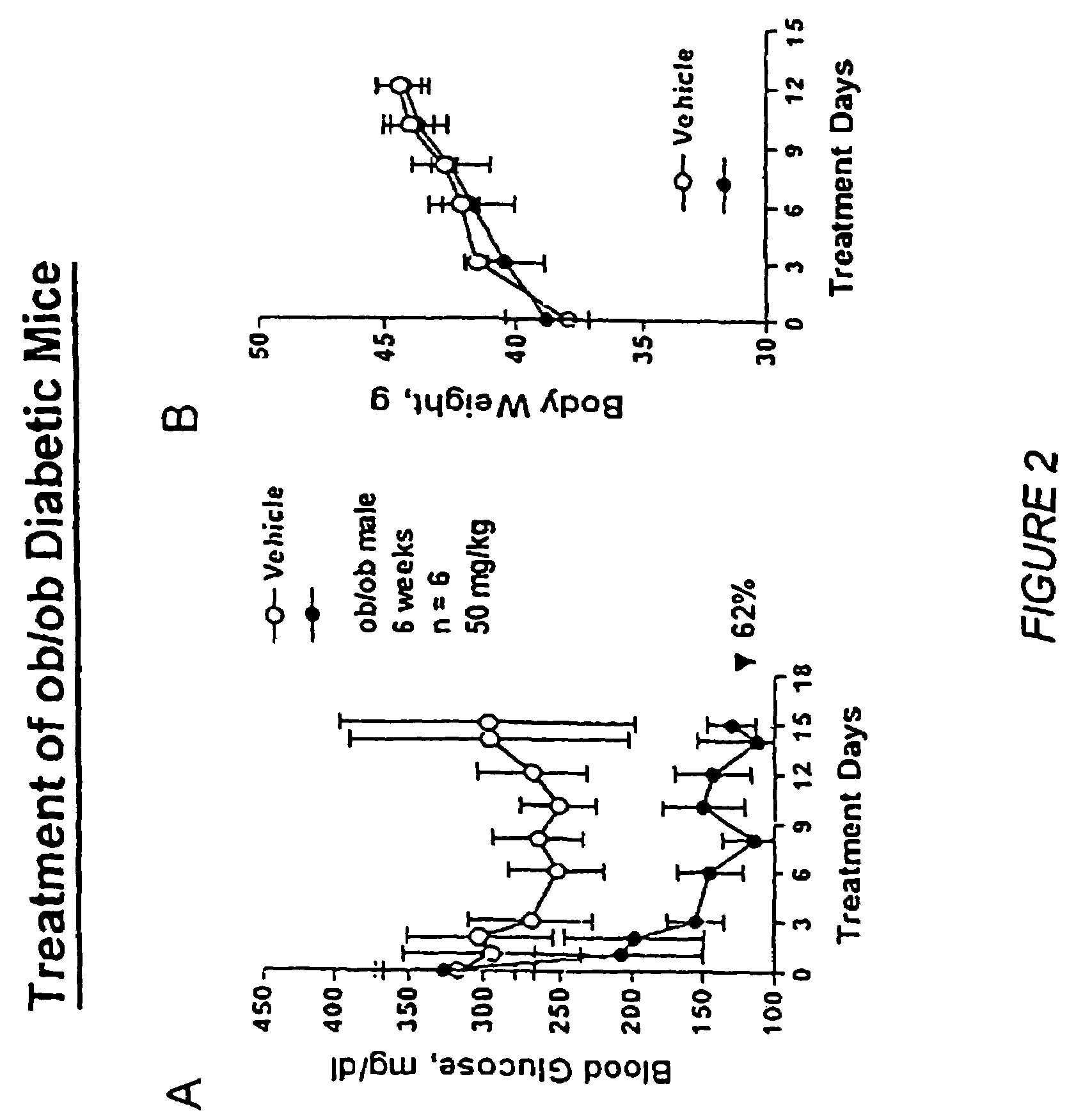
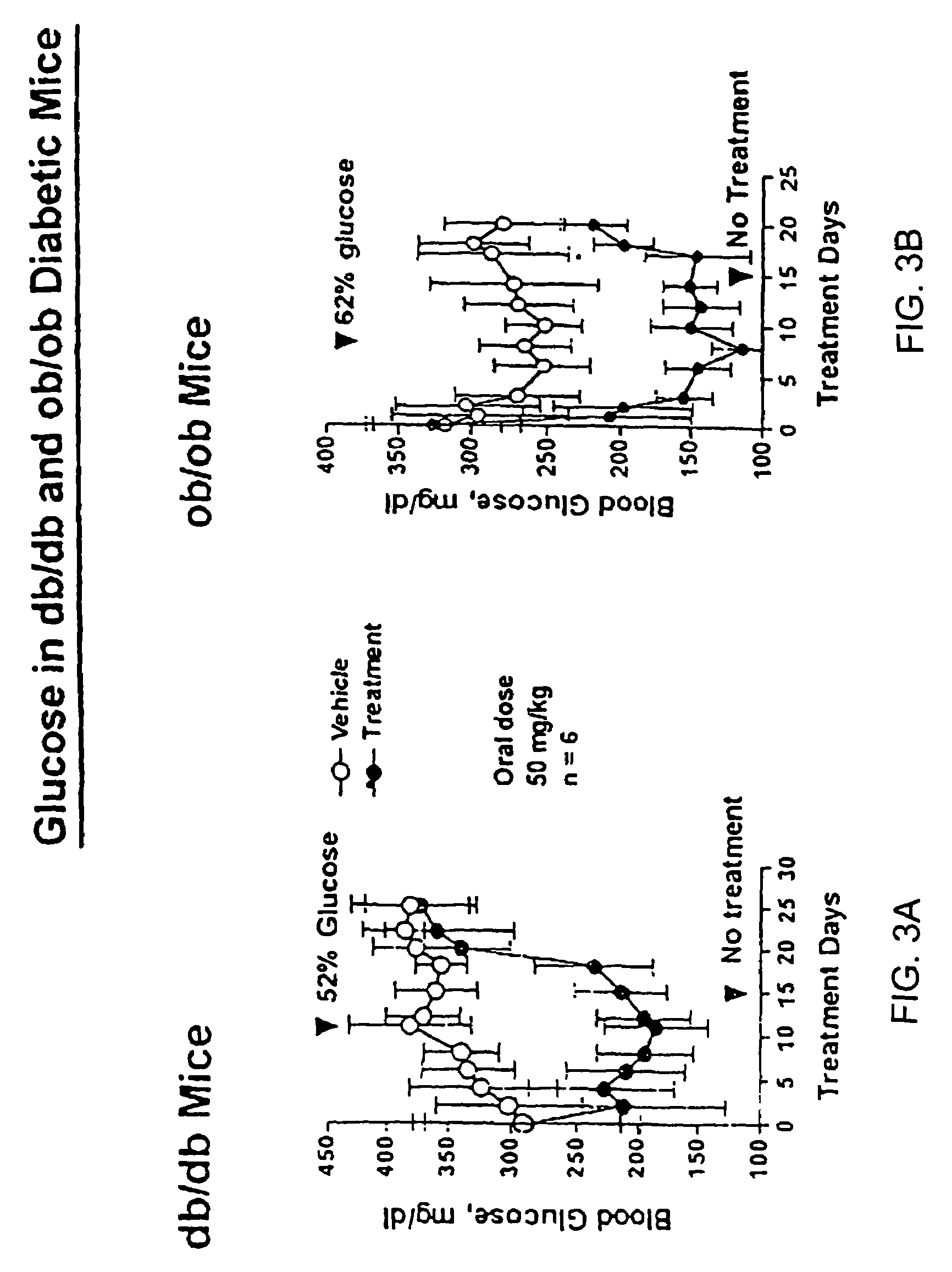
Oxazolidinedione is a heterocyclic chemical compound that forms the core structure of a variety anticonvulsant drugs including...
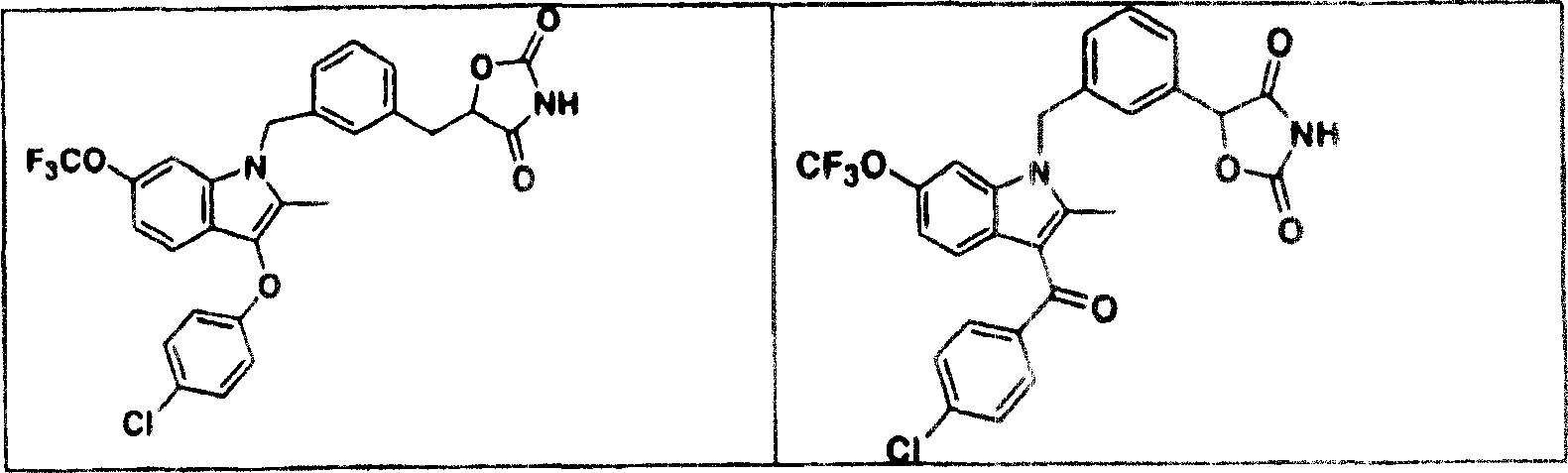
Novel heterocyclic analogs of diphenylethylene compounds
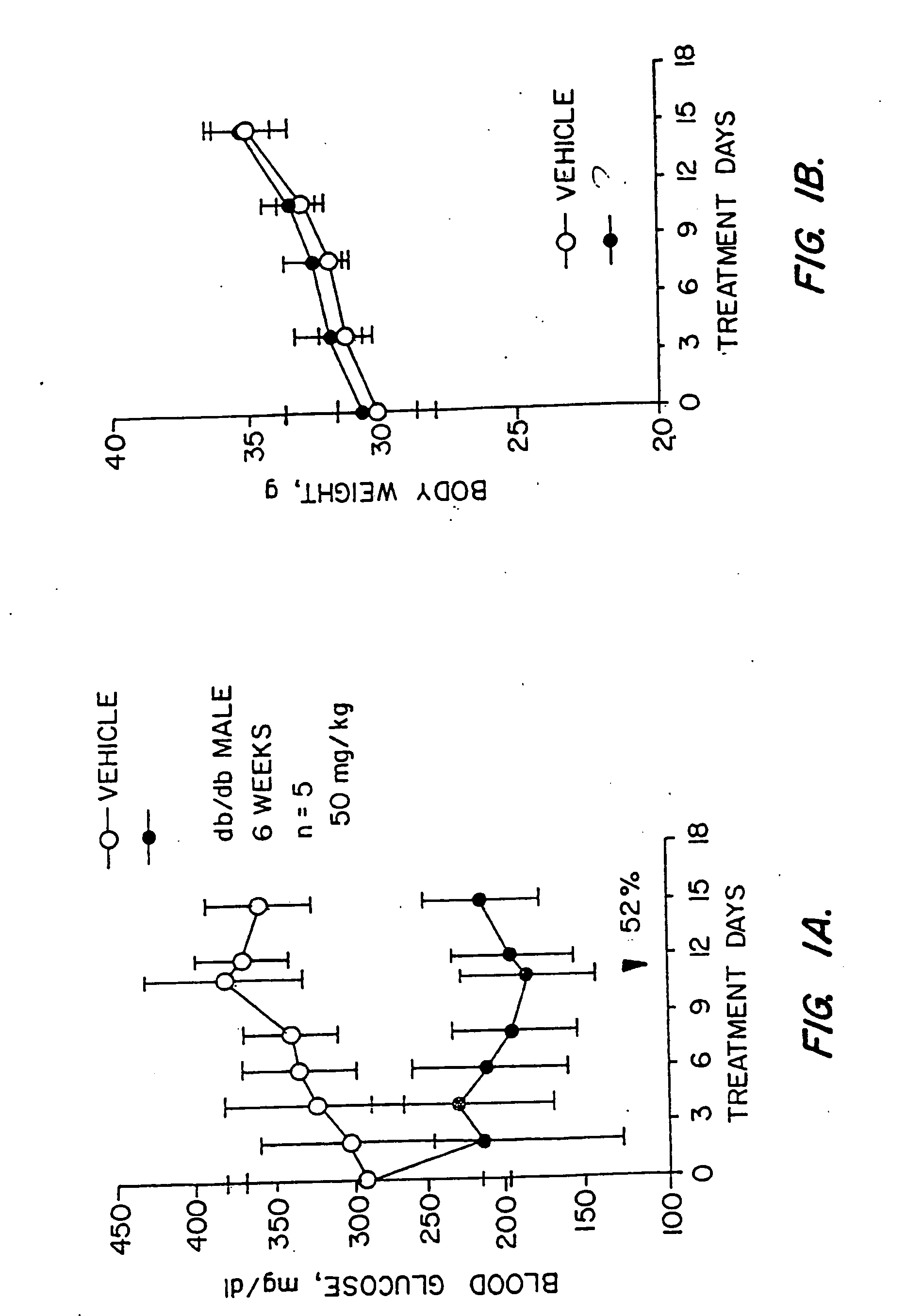
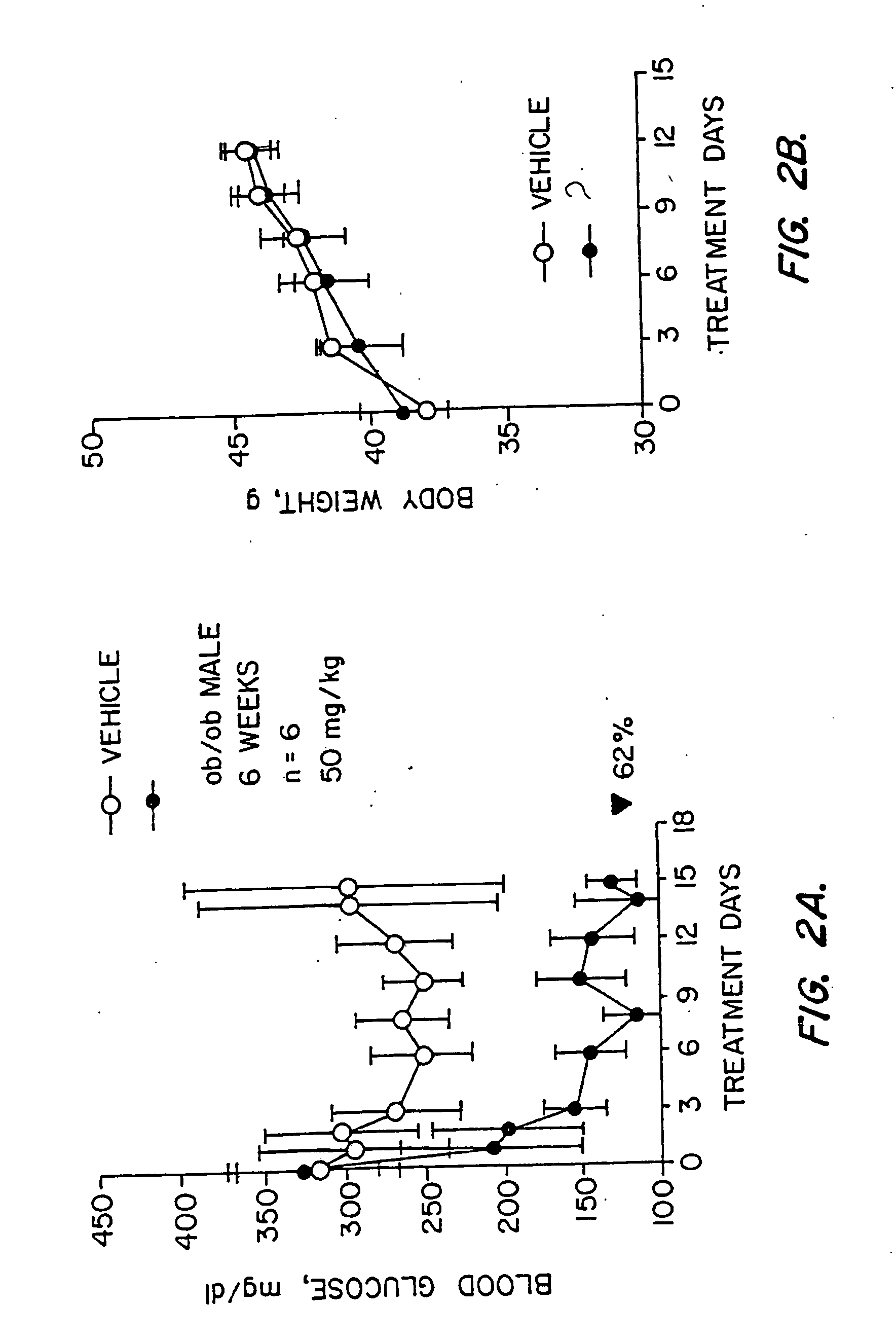
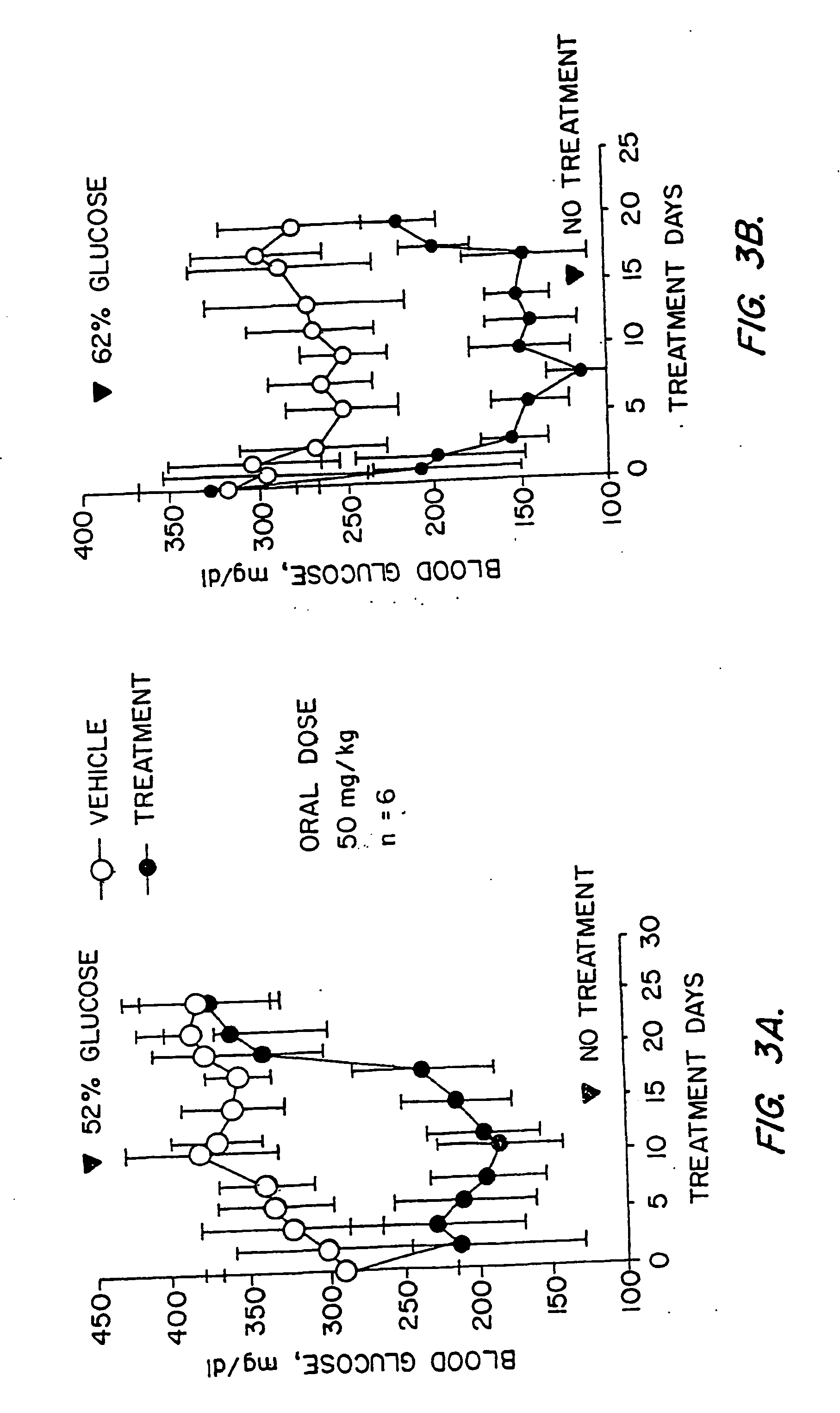
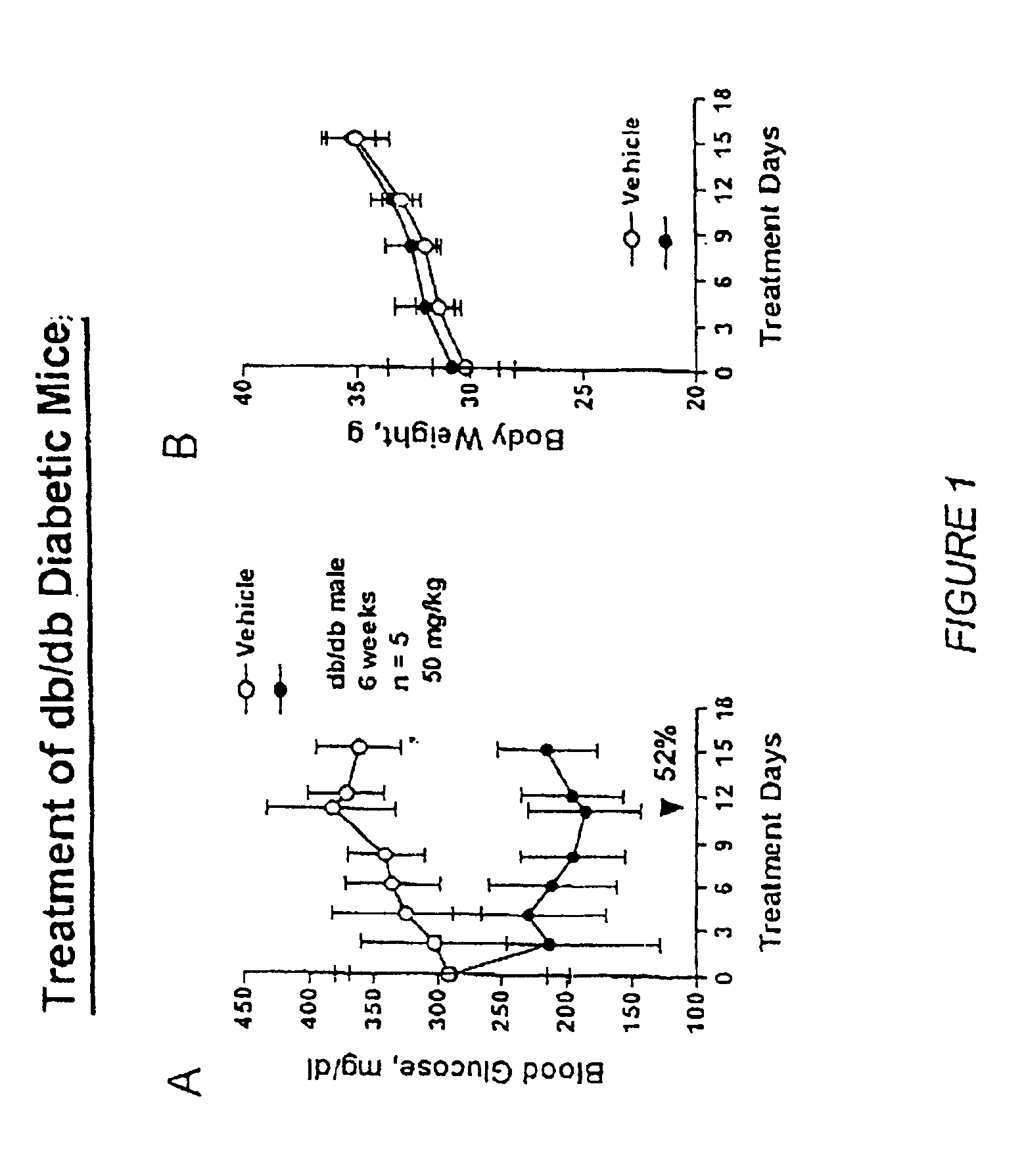
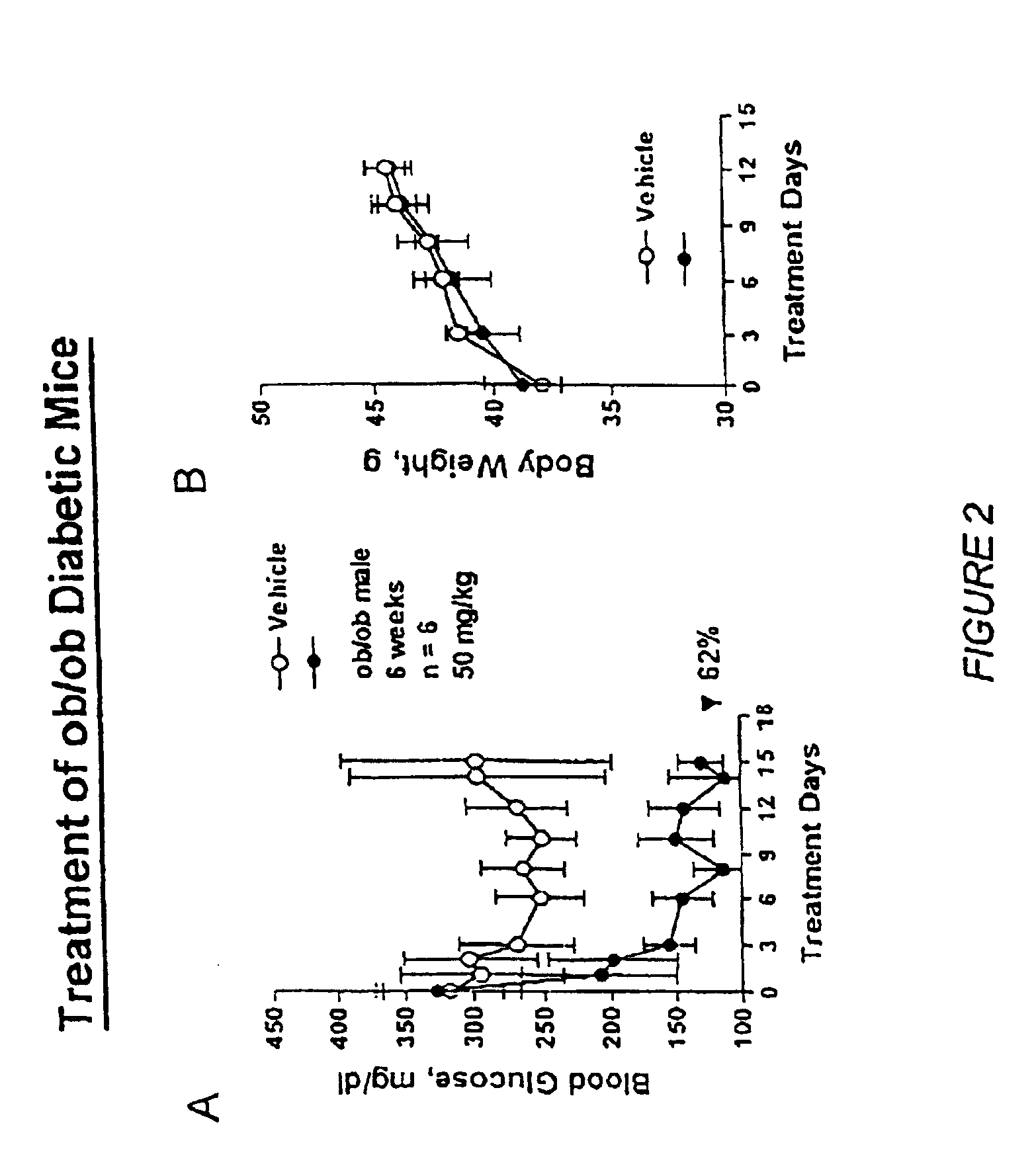
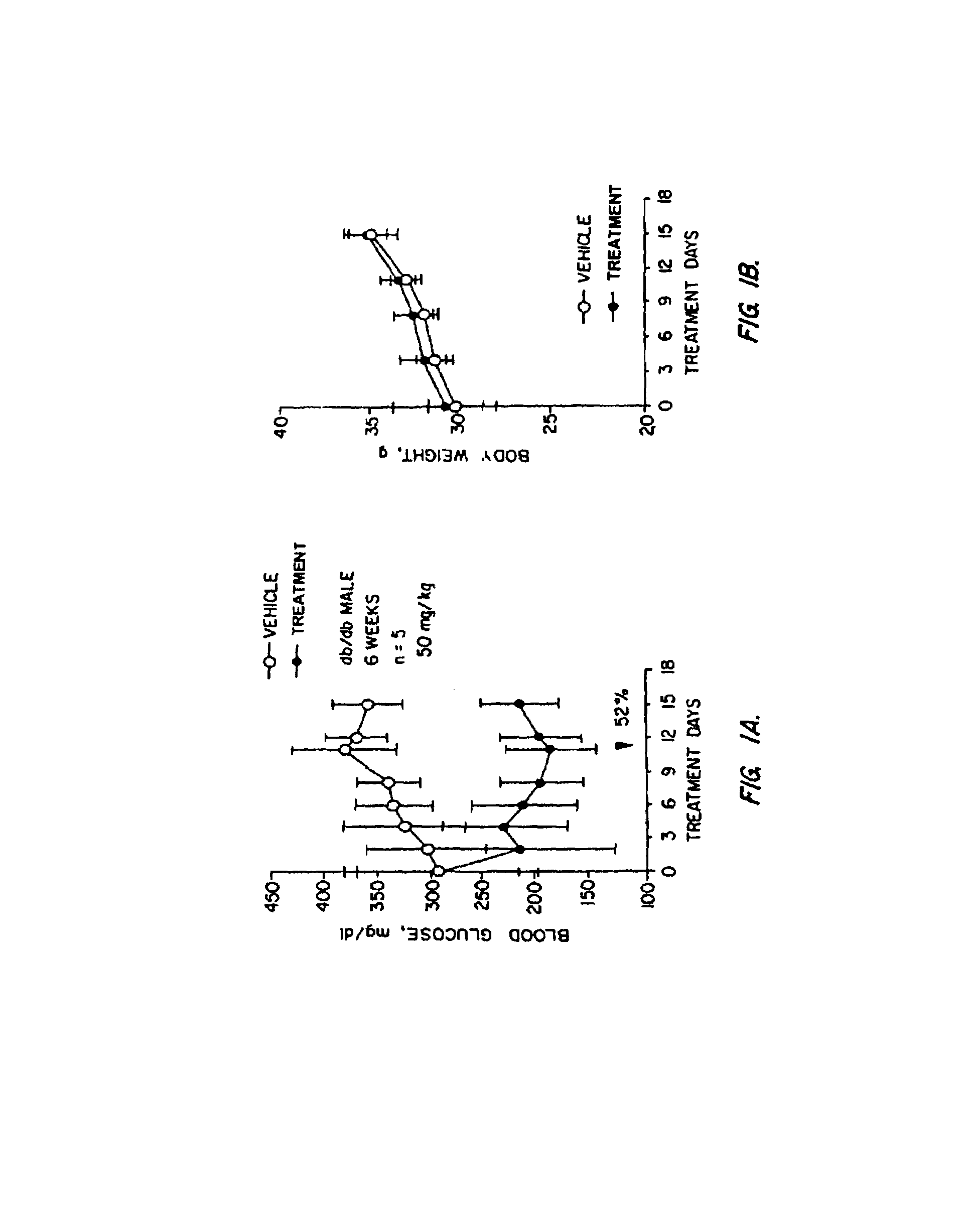
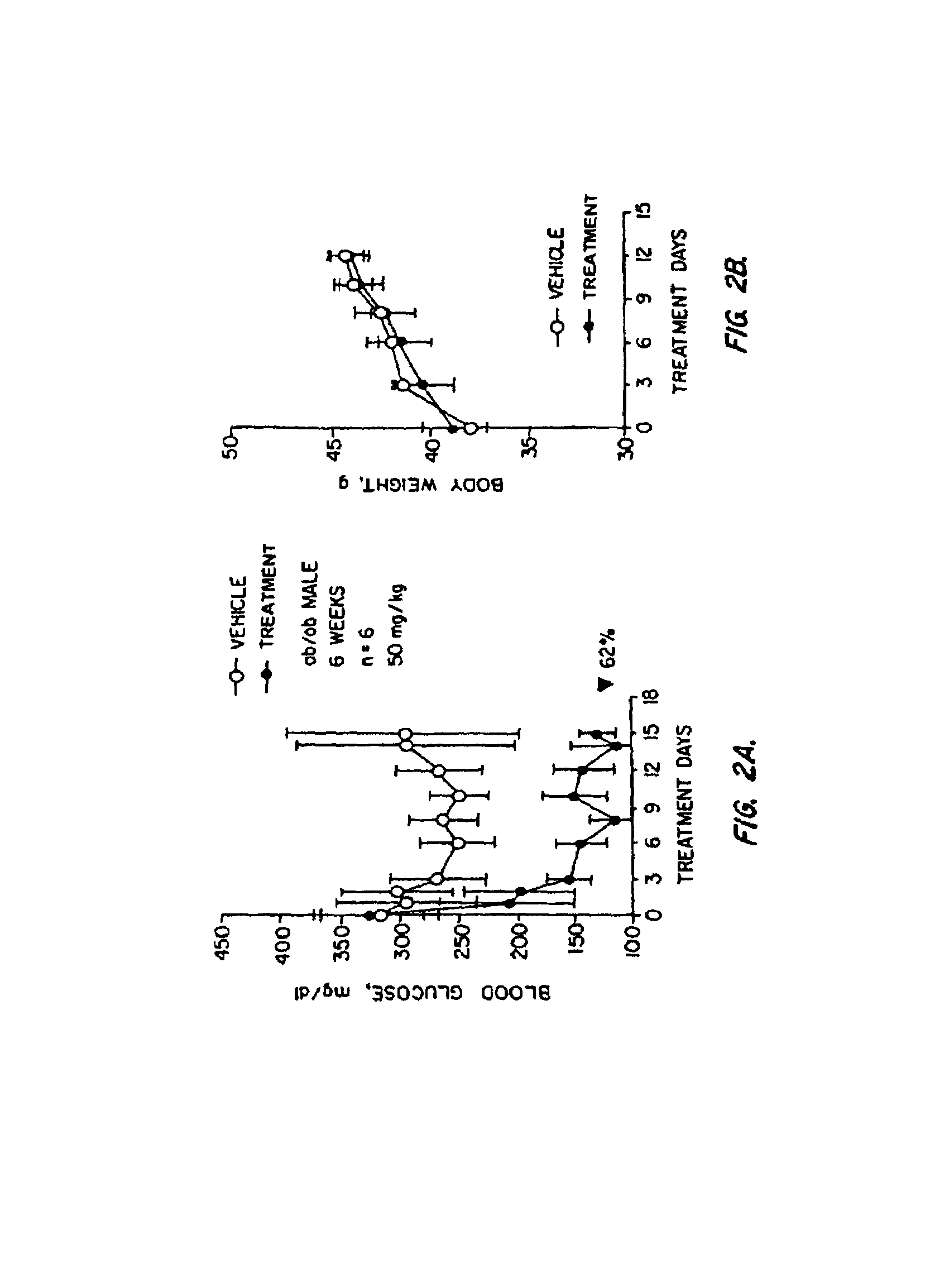
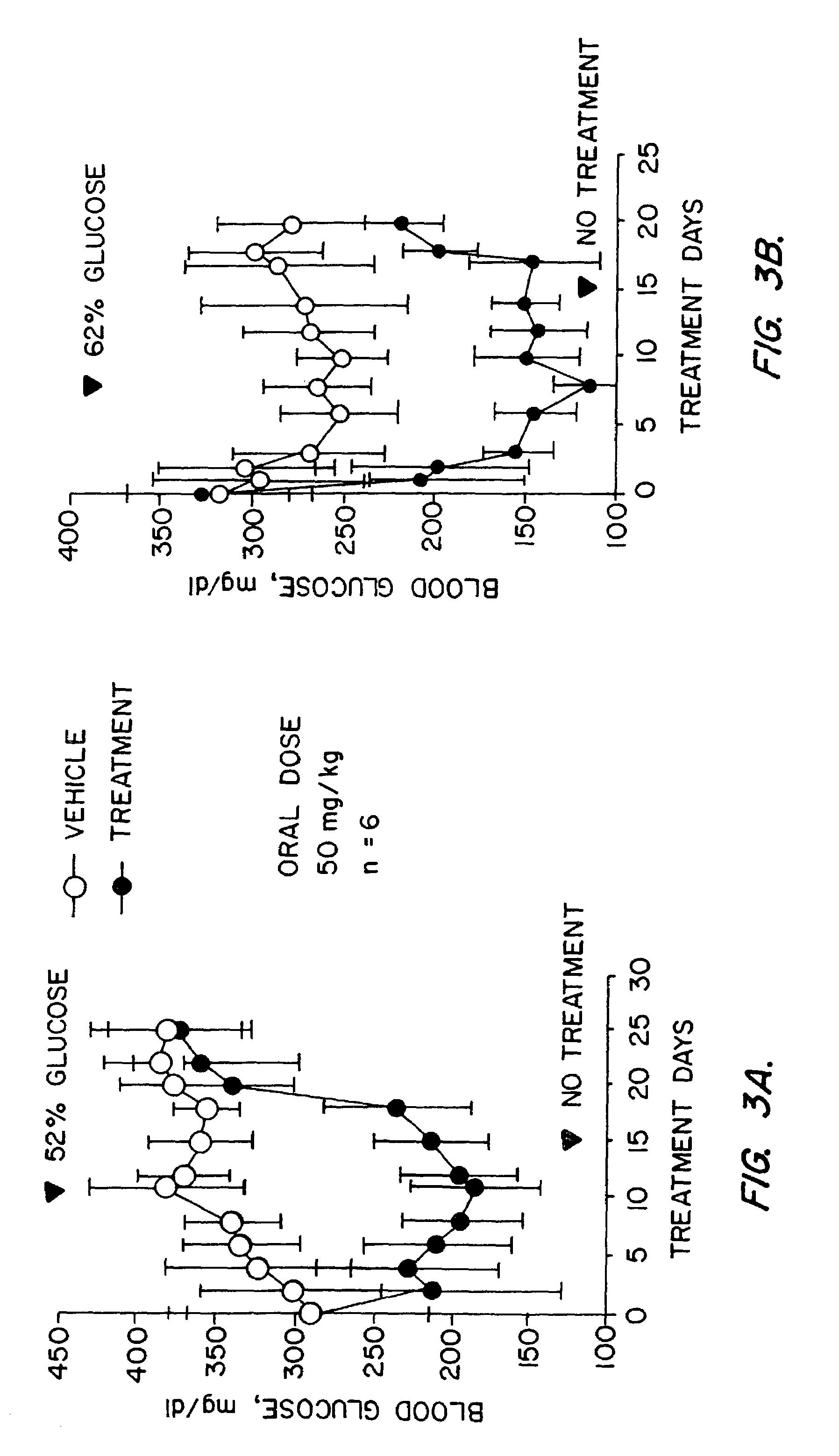
Novel diphenylethylene compounds and derivatives thereof containing thiazolidinedione or oxazolidinedione moieties are provided which are effective in lowering blood glucose level, serum insulin, triglyceride and free fatty acid levels in animal models of Type II diabetes. The compounds are disclosed as useful for a variety of treatments including the treatment of inflammation, inflammatory and immunological diseases, insulin resistance, hyperlipidemia, coronary artery disease, cancer and multiple sclerosis.
Owner:THERAKOS INC
Thiazolidinedione, oxazolidinedione and oxadiazolidinedione derivatives
Novel thiazolidinedione, oxazolidinedione and oxadiazolidinedione derivatives, process for their manufacture, pharmaceutical preparations containing them and the use of the compounds in conditions associated with insulin resistance.
Owner:ASTRAZENECA AB
Heterocyclic analogs of diphenylethylene compounds
Novel diphenylethylene compounds and derivatives thereof containing thiazolidinedione or oxazolidinedione moieties are provided which are effective in lowering blood glucose level, serum insulin, triglyceride and free fatty acid levels in animal models of Type II diabetes. In contrast to previously reported thiazolidinedione compounds, known to lower leptin levels, the present compounds increase leptin levels and have no known liver toxicity. The compounds are disclosed as useful for a variety of treatments including the treatment of inflammation, inflammatory and immunological diseases, insulin resistance, hyperlipidemia, coronary artery disease, cancer and multiple sclerosis.
Owner:THERAKOS INC
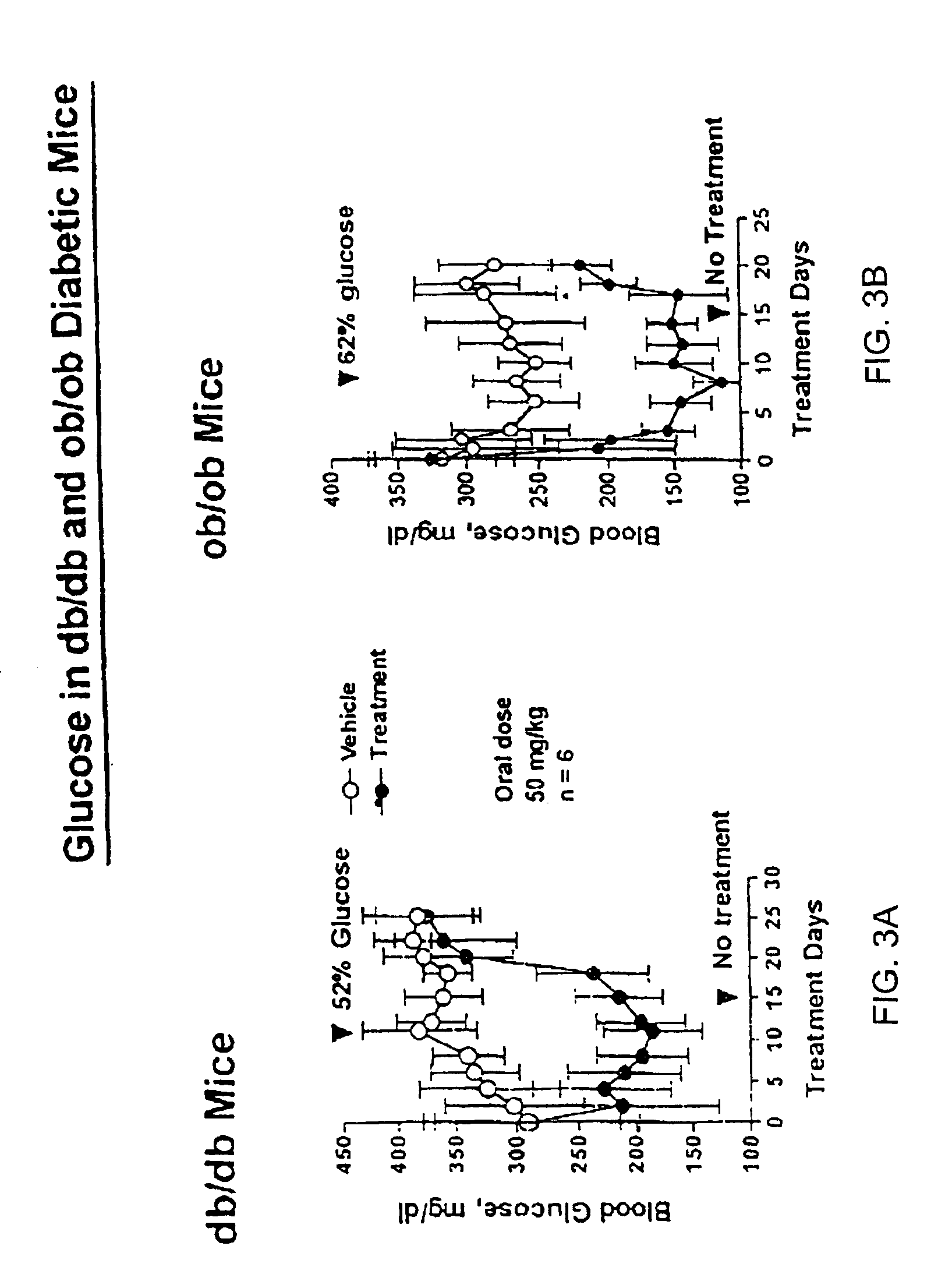
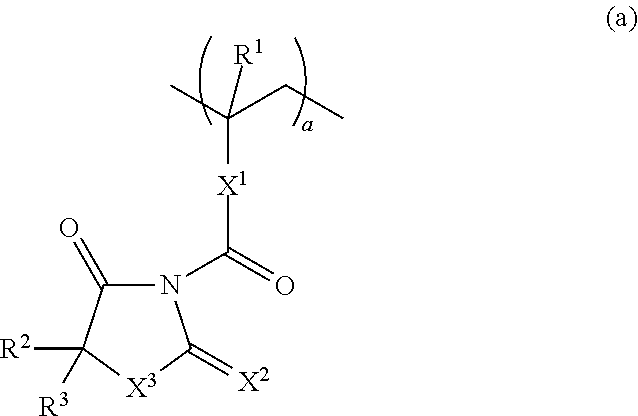
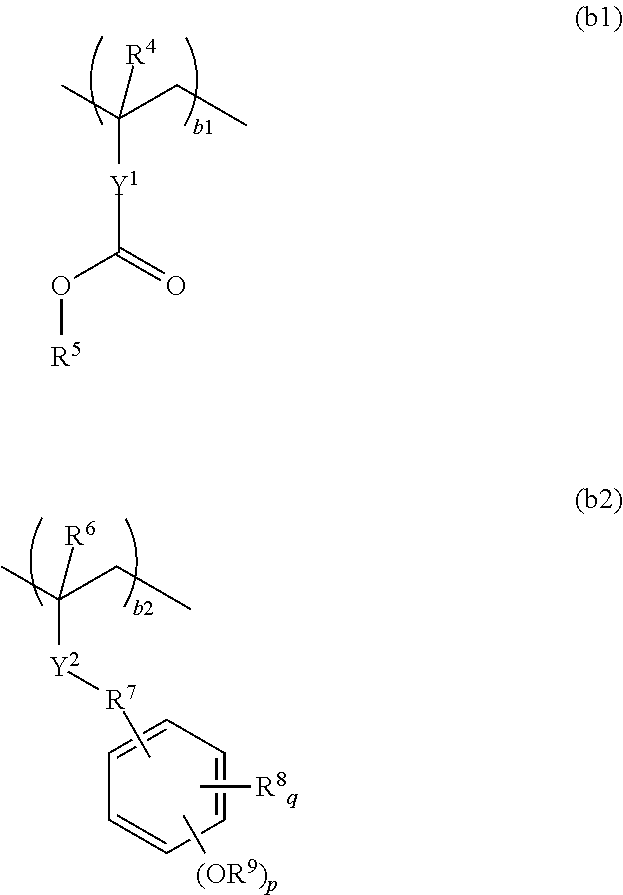
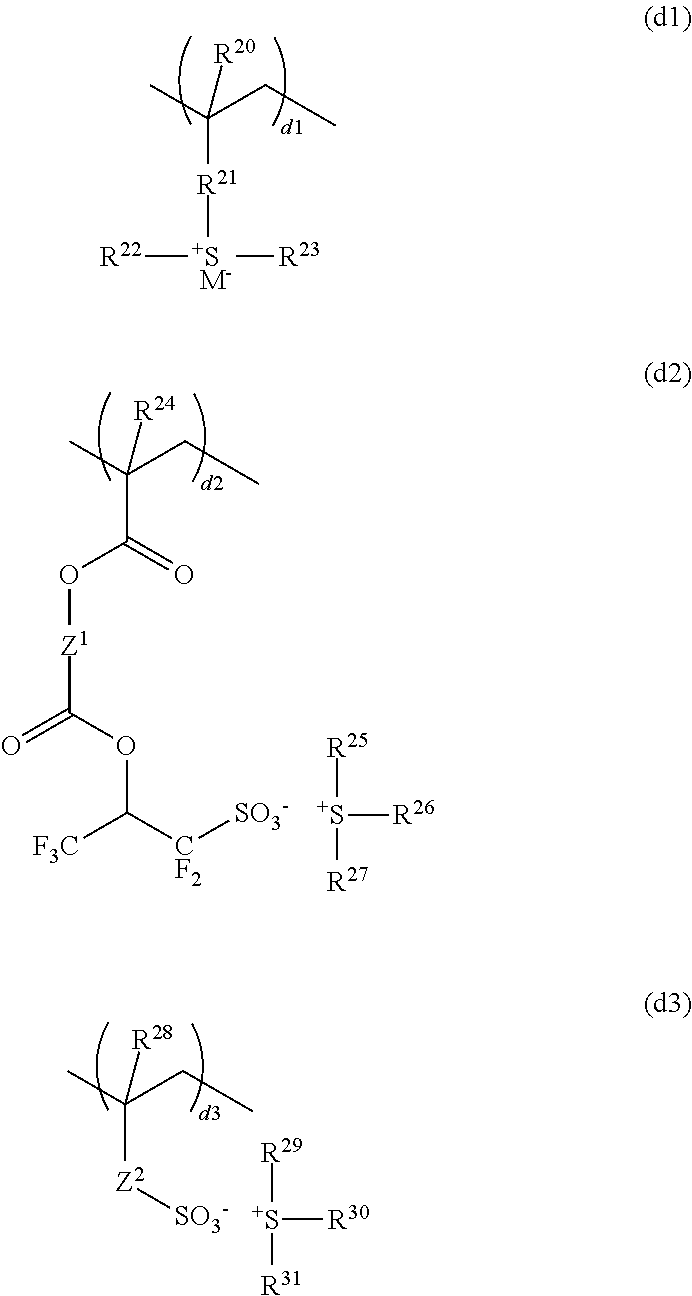
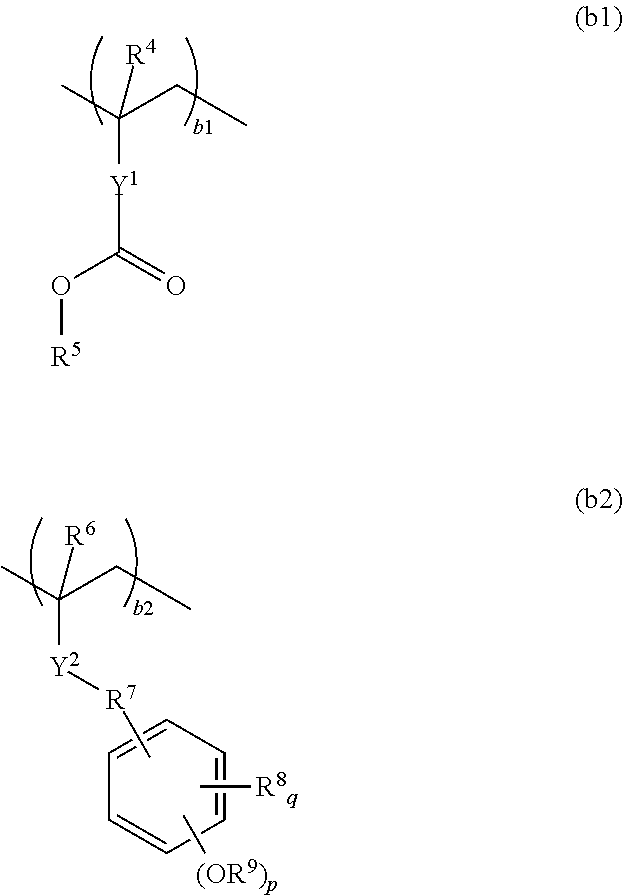
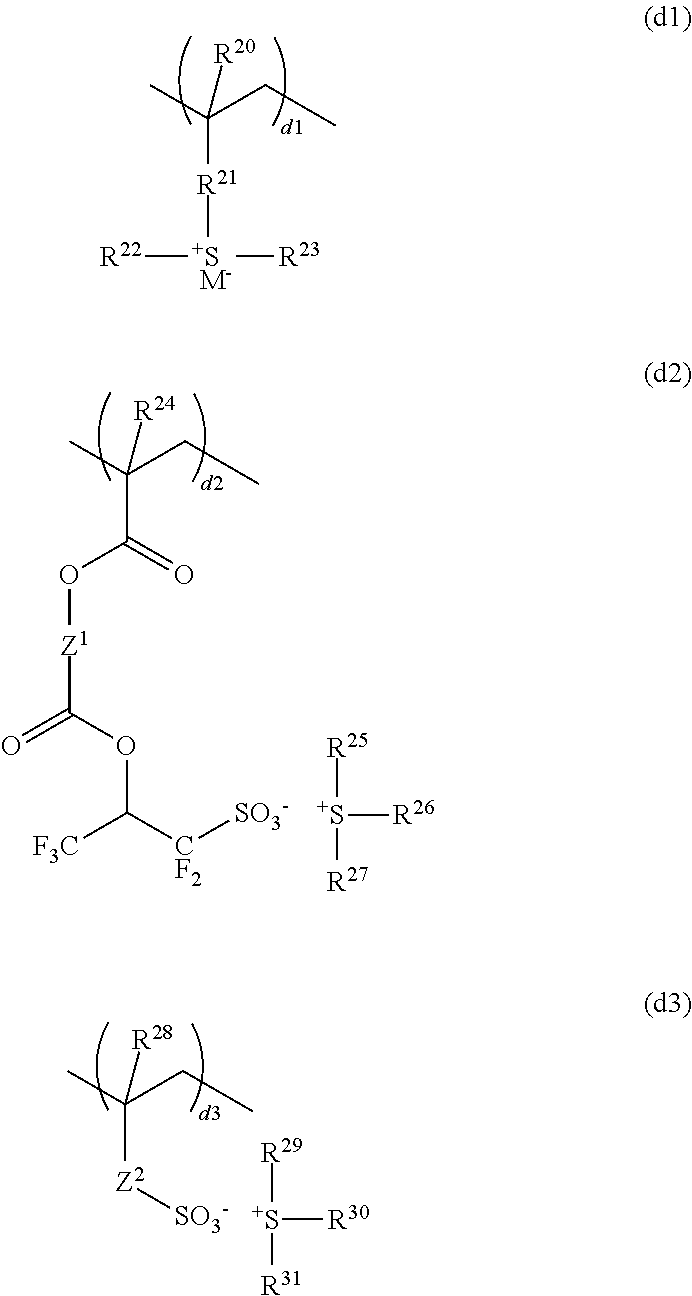
Resist composition and pattern forming process
ActiveUS20170031243A1High resolutionMinimal edge roughnessPhotomechanical coating apparatusPhotomechanical exposure apparatusResistOxazolidinedione
A resist composition is provided comprising a polymer comprising recurring units (a) having an oxazolidinedione, thioxooxazolidinone, thiazolidinedione or thioxothiazolidinone structure and recurring unit (b1) having an acid labile group-substituted carboxyl group and / or recurring units (b2) having an acid labile group-substituted phenolic hydroxyl group. The resist composition suppresses acid diffusion, exhibits a high resolution, and forms a pattern of satisfactory profile with low edge roughness.
Owner:SHIN ETSU CHEM IND CO LTD
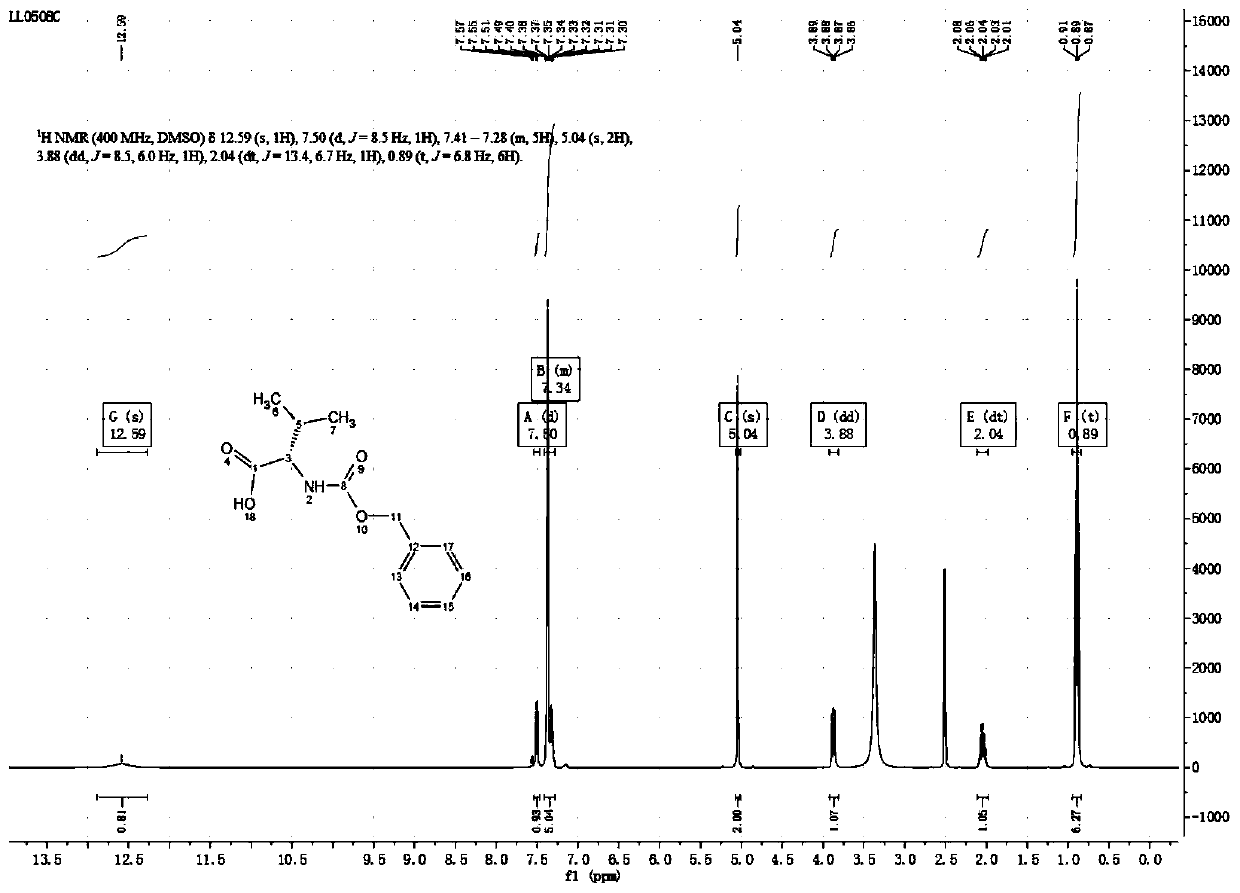
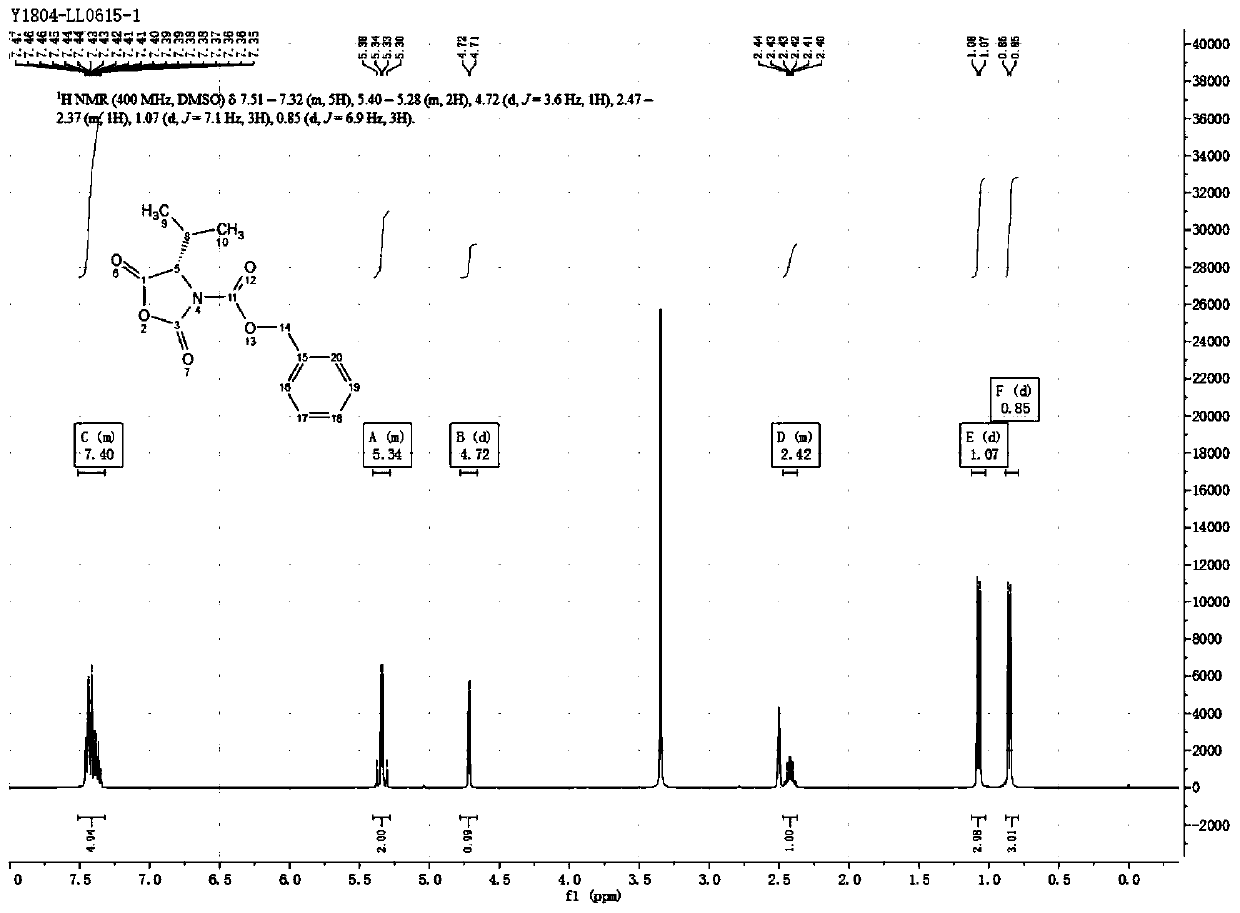
Preparation technology of (S)-3-carbobenzoxy-4-isopropyl-2,5-oxazolidinedione
ActiveCN110204505AHigh yieldLow yieldOrganic chemistry methodsBulk chemical productionOxazolidinedioneValganciclovir Hydrochloride
The invention provides a preparation technology of (S)-3-carbobenzoxy-4-isopropyl-2,5-oxazolidinedione. The (S)-3-carbobenzoxy-4-isopropyl-2,5-oxazolidinedione can serve as a valganciclovir hydrochloride intermediate. The technology includes the following operations that an L-valine starting material reacts with benzyl chloroformate to generate N-carbobenzoxy-L-valine; the N-carbobenzoxy-L-valinereacts with N,N-carbonyl diimidazole (CDI) to generate the (S)-3-carbobenzoxy-4-isopropyl-2,5-oxazolidinedione. The preparation technology is simple in method, purification is easy, the process is stable, the quality is controllable, the product yield is greatly improved, environmental pollution is not caused, and the technology is suitable for industrial mass production.
Owner:荆门医药工业技术研究院 +1
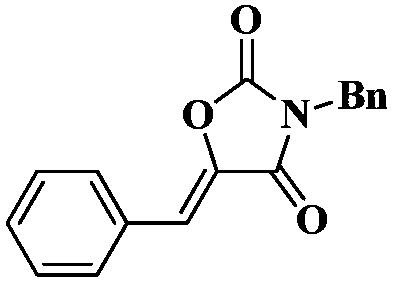
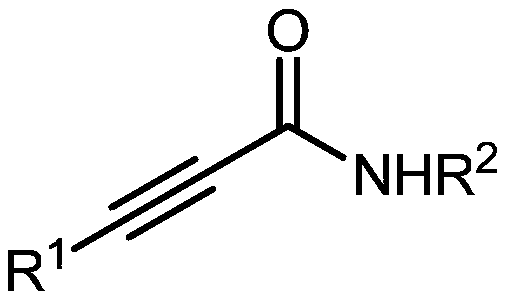
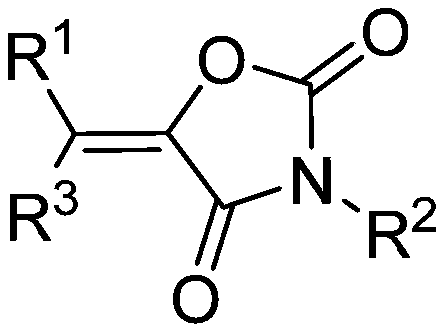
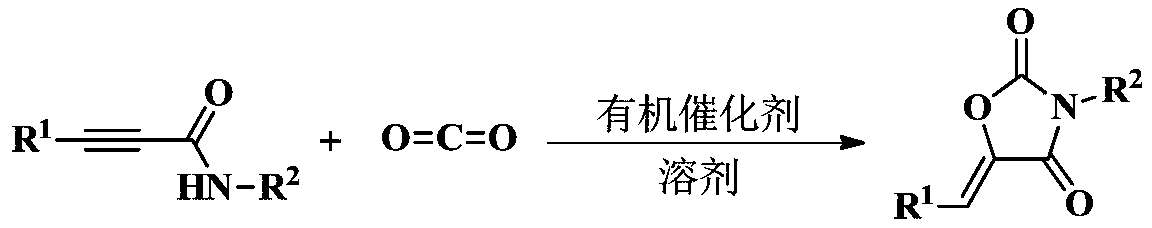
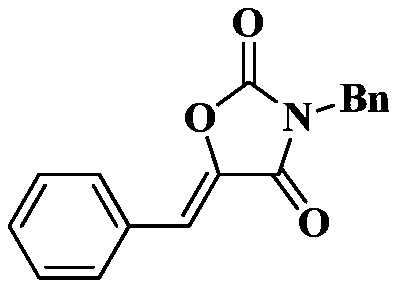
Method for directly synthesizing oxazolidin-2,4-one heterocyclic compound from alkyne amide
ActiveCN111943901AMild reaction conditionsEasy to operateOrganic chemistryOxazolidinedioneTetrafluoroborate
The invention discloses a novel method for directly synthesizing an oxazolidin-2,4-one heterocyclic compound from an alkyne amide raw material. According to the method, Bronsted acid or tetrafluoroborate with a molar fraction of 10% is used as a catalyst, nitromethane, N-methylpyrrolidone, dimethyl sulfoxide or N,N-dimethylformamide is used as a solvent a reaction is carried out for 0.5-24 h at atemperature of 50-150 DEG C under the condition ofair,then vacuum concentration is carried out, and recrystallization or column chromatography purification is carried out, so the oxazolidin-2,4-one heterocyclic compound is obtained. The yield of the oxazolidin-2,4-one heterocyclic compound is 89-92%. The method has high catalytic reaction activity, realizes the preparation of the oxazolidin-2,4-one heterocyclic compound from an alkyne amide raw material for the first time, is high in yield, wide in the range of applicable substrates and capable of synthesizing the oxazolidin-2,4-one heterocyclic compound on a gram-level scale, and has the advantages of insensitivity to air, high chemical selectivity, easiness in product separation, short reaction time and the like.
Owner:NANJING TECH UNIV
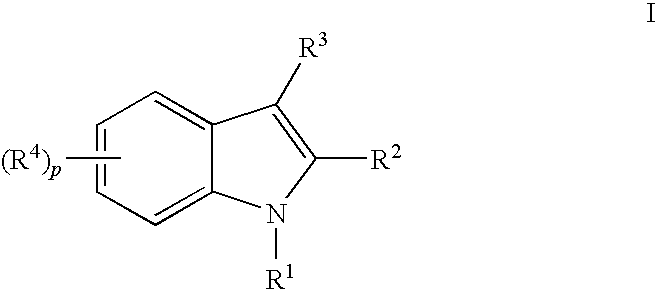
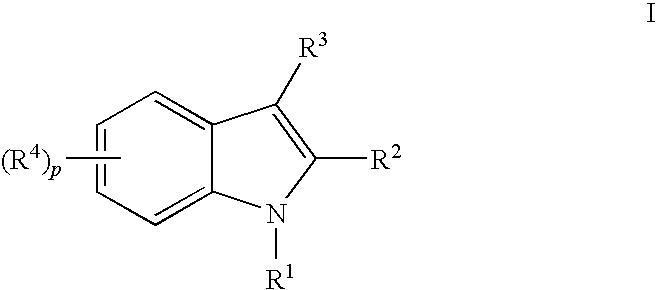
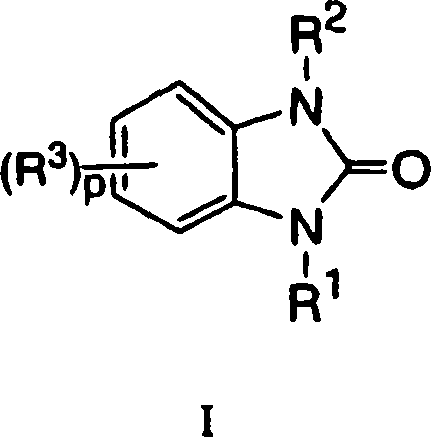
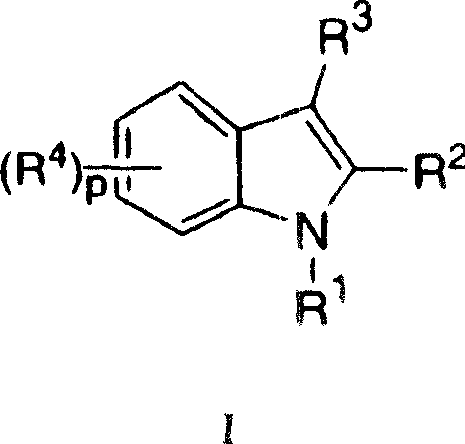
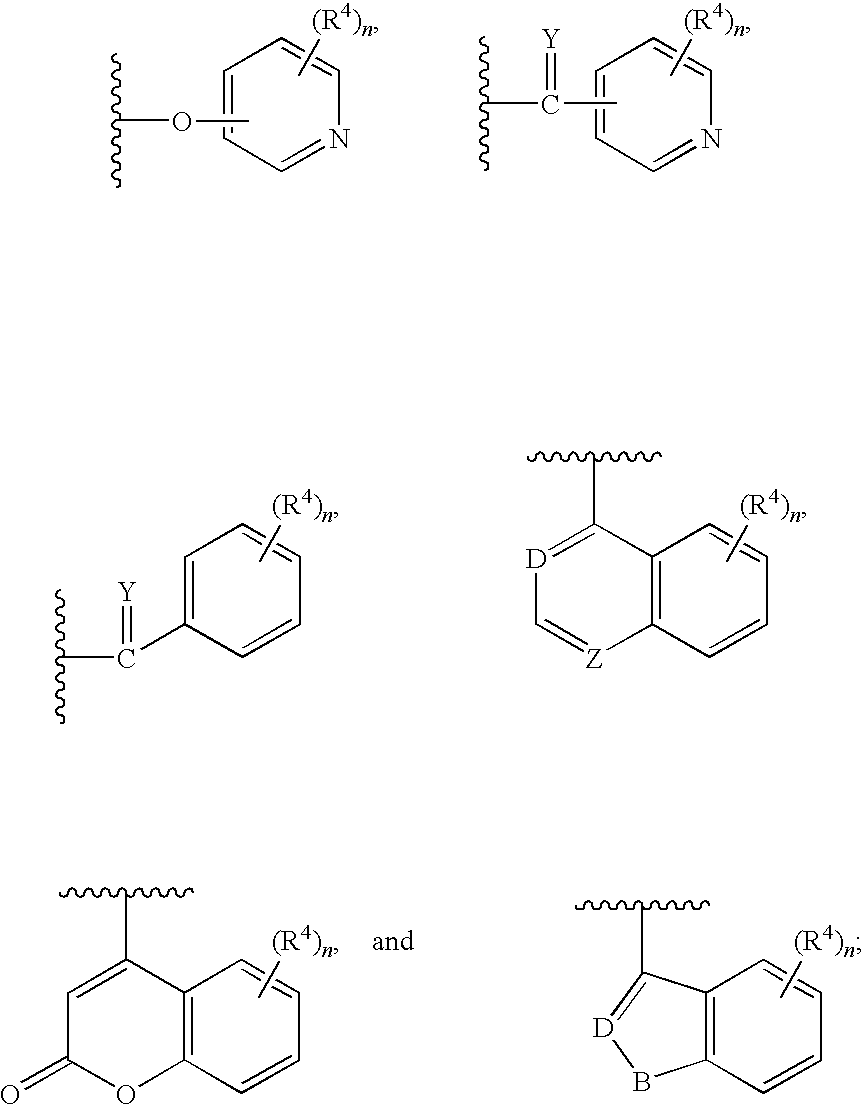
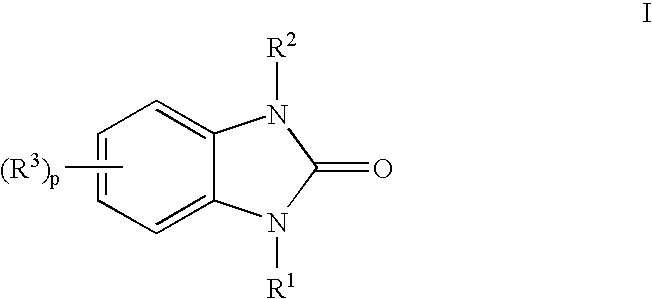
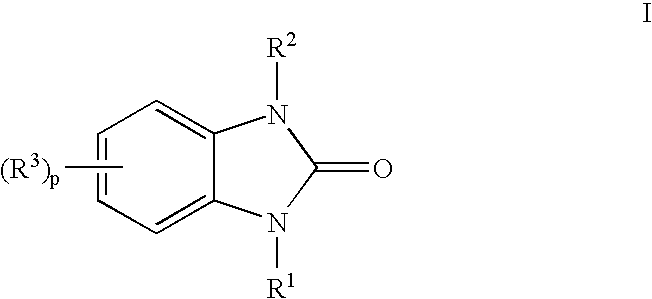
Indoles having anti-diabetic activity
Indoles of Formula I having —X-aryl-(CH2)x-oxazolidinedione and —X-heteroaryl-(CH2)x-oxazolidinedione substituents on the N atom of the indole ring, where x is 0 or 1, and —X— is a bond or —CH2—, and their thiazolidinedione analogs, are PPAR gamma agonists or partial agonists and are useful in the treatment and control of type II diabetes, including hyperglycemia, dyslipidemia, hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, and obesity that are often associated with type 2 diabetes.
Owner:MERCK SHARP & DOHME CORP
Benzoureas having anti-diabetic activity
Benzourea compounds of Formula I having aryl-(CH2)x-oxazolidinedione or aryl-(CH2)x-thiazolidinedione substituents on one of the N atoms of the benzourea ring, wherein x is 0 or 1, are PPAR gamma agonists or partial agonists and are useful in the treatment and control of type II diabetes, including hyperglycemia and other symptoms such as dyslipidemia, hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, and obesity, that are often associated with type 2 diabetes.
Owner:MERCK & CO INC
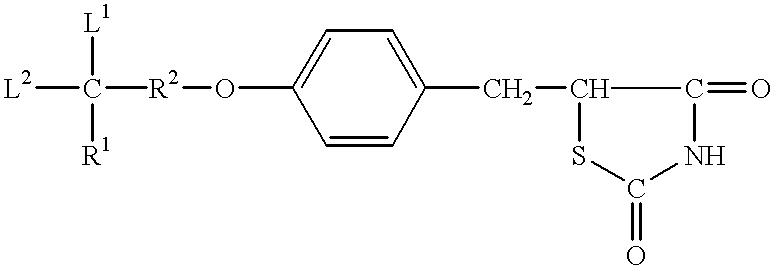
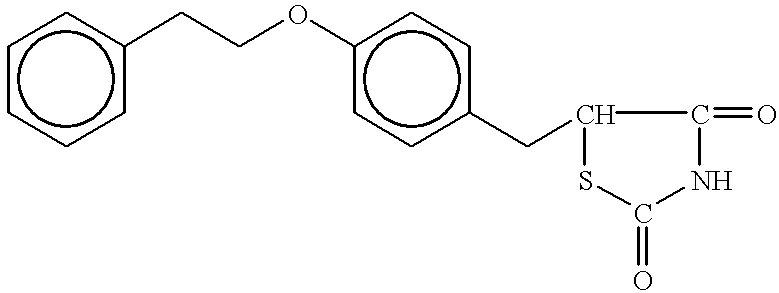
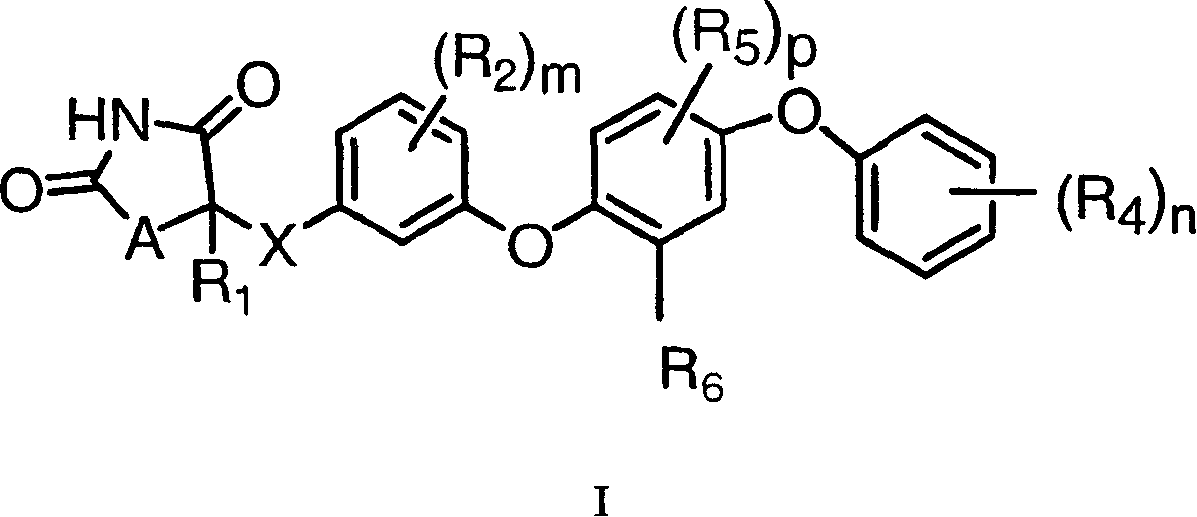
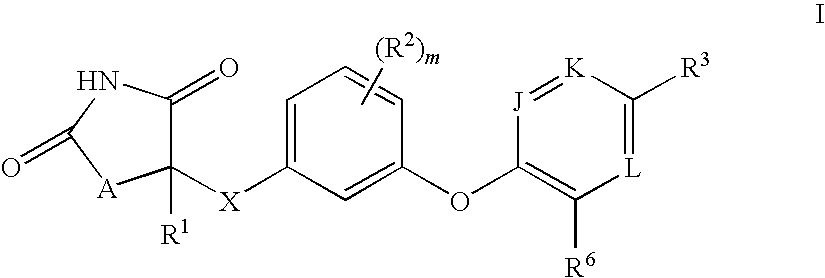
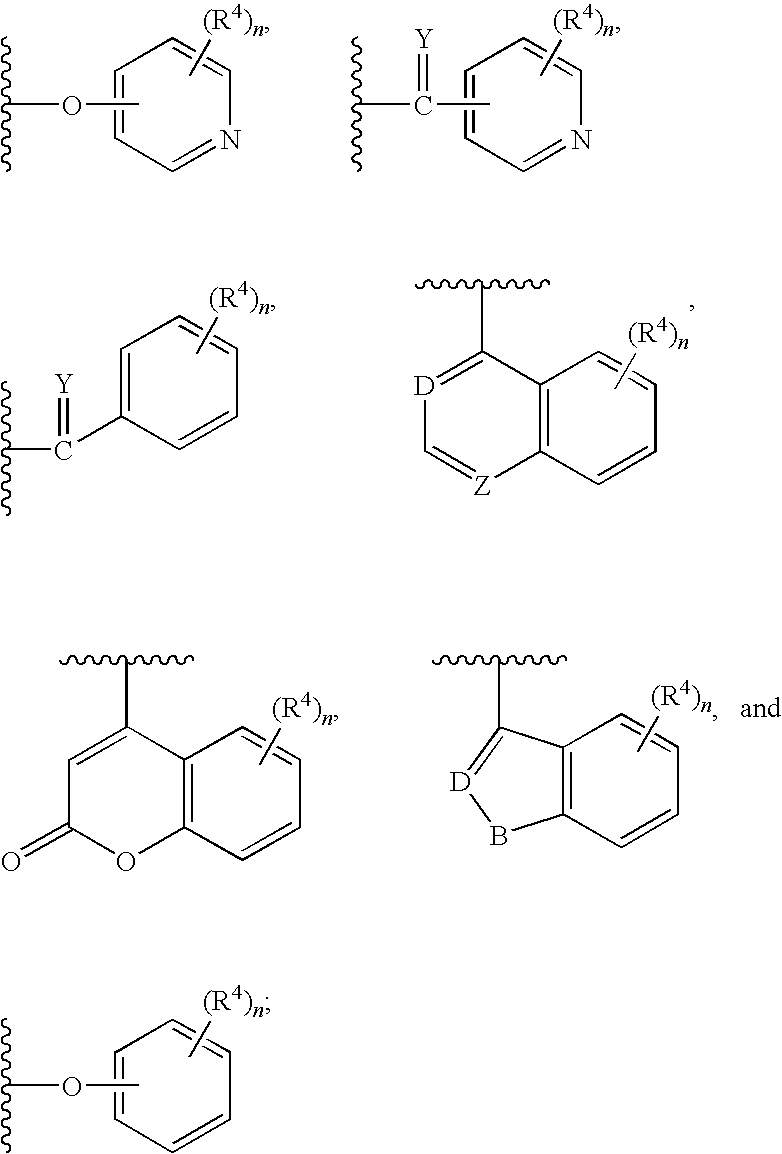
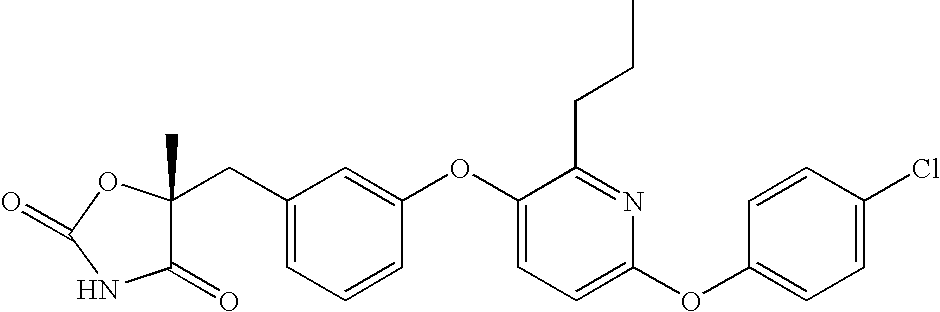
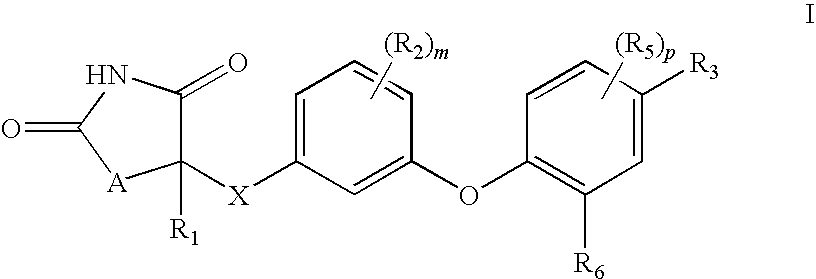
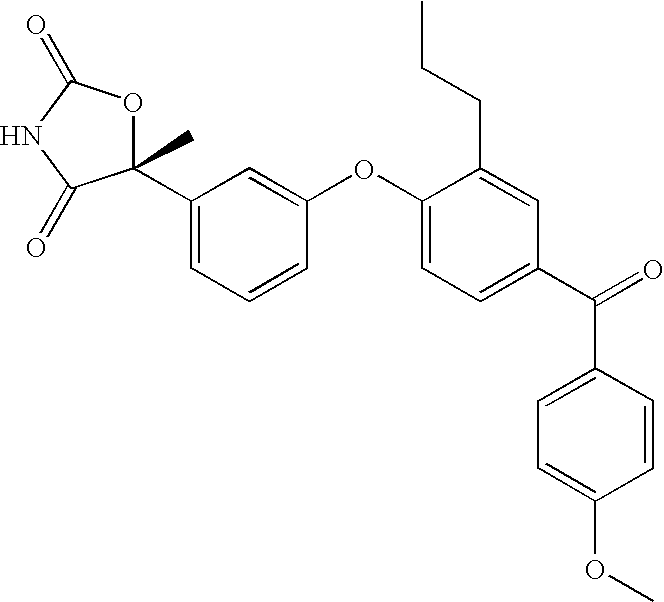
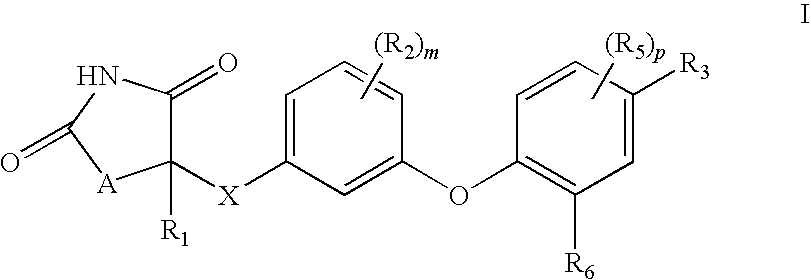
Antidiabetic oxazolidinediones and thiazolidinediones
Phenoxyphenyl and phenoxybenzyl oxazolidine-2,4-diones and thiazolidine-2,4-diones of formula (I) are agonists or partial agonists of PPAR gamma and are useful in the treatment and control of hyperglycemia that is symptomatic of type II diabetes, as well as dyslipidemia, hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, and obesity that are often associated with type 2 diabetes.
Owner:MERCK & CO INC
Method used for synthesizing 2, 4-oxazolidinedione compound through organic amine catalyzing CO2
The invention provides a method used for synthesizing 2, 4-oxazolidinedione compound through organic amine catalyzing CO2, and belongs to the technical field of organic synthesis, pesticide, and medical chemistry. The method comprises following steps: acetylene amide raw material and a solvent are introduced into a reaction bottle containing a magneton, an organic base is added as a catalyst, thereaction bottle is introduced into a Schlink bottle filled with carbon dioxide gas, stirring reaction is carried out for 1 to 6h at room temperature, after reaction, an obtained reaction liquid is pumped out from the reaction bottle, an obtained crude product is subjected to column chromatography so as to obtain the 2, 4-oxazolidinedione compound. According to the method, the simple organic base of a catalytic amount is adopted to replace a conventional metal catalyst; reaction is clean; reaction conditions are mild; function group tolerance is high; conversion rate and stereoselectivity are high, and the application prospect in the fields of organic synthesis, pesticide, and medicine is promising.
Owner:DALIAN UNIV OF TECH
Indoles having anti-diabetic activity
Owner:MERCK & CO INC
Heterocyclic analogs of diphenylethylene compounds
Novel diphenylethylene compounds and derivatives thereof containing thiazolidinedione or oxazolidinedione moieties are provided which are effective in lowering blood glucose level, serum insulin, triglyceride and free fatty acid levels in animal models of Type II diabetes. In contrast to previously reported thiazolidinedione compounds, known to lower leptin levels, the present compounds increase leptin levels and have no known liver toxicity. The compounds are disclosed as useful for a variety of treatments including the treatment of inflammation, inflammatory and immunological diseases, insulin resistance, hyperlipidemia, coronary artery disease, cancer and multiple sclerosis.
Owner:THERAKOS INC
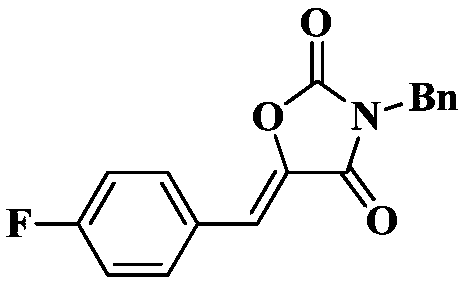
Method for synthesizing 3-(2-phenylethyl)-5-[2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolin-en]-2,4-oxazolidinedione
InactiveCN101121714ASimple preparation processRaw materials are cheap and easy to getOrganic chemistryOxazolidinedioneSynthesis methods
The invention discloses a synthesis method of the 3-(2-phenyl ethyl)-5-[2, 3, 6, 7-tetrahydrogen-1H, 5H-dibenzo [ij] quinoline-9-ene]-2, 4-oxazolidine dione; the steps first uses the hydroxy ethyl acetate and urea as the raw materials to make the oxazolidine-2 ,4-dione; then the oxazolidine-2 ,4-dione reacts with the beta-bromophenyl ethane to make the 3-phenethyl-oxazolidine-2, 4-dione; the 3-phenethyl-oxazolidine-2, 4-dione then reacts with the 2,3,6,7-tetrahydrogen-1 H, 5H-pyrido [3,2,1-ij] quinoline-9-formaldehyde to make the product 3-(2-phenyl ethyl)-5-[2,3,6, 7-tetrahydrogen-1 H, 5H-benzo [ij] quinoline-9-ene]-2,4-oxazolidine dione. The invention is of the simple process, the easily accessible and cheap raw materials, the high production rate, the low cost and the suitableness for the industrial production.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
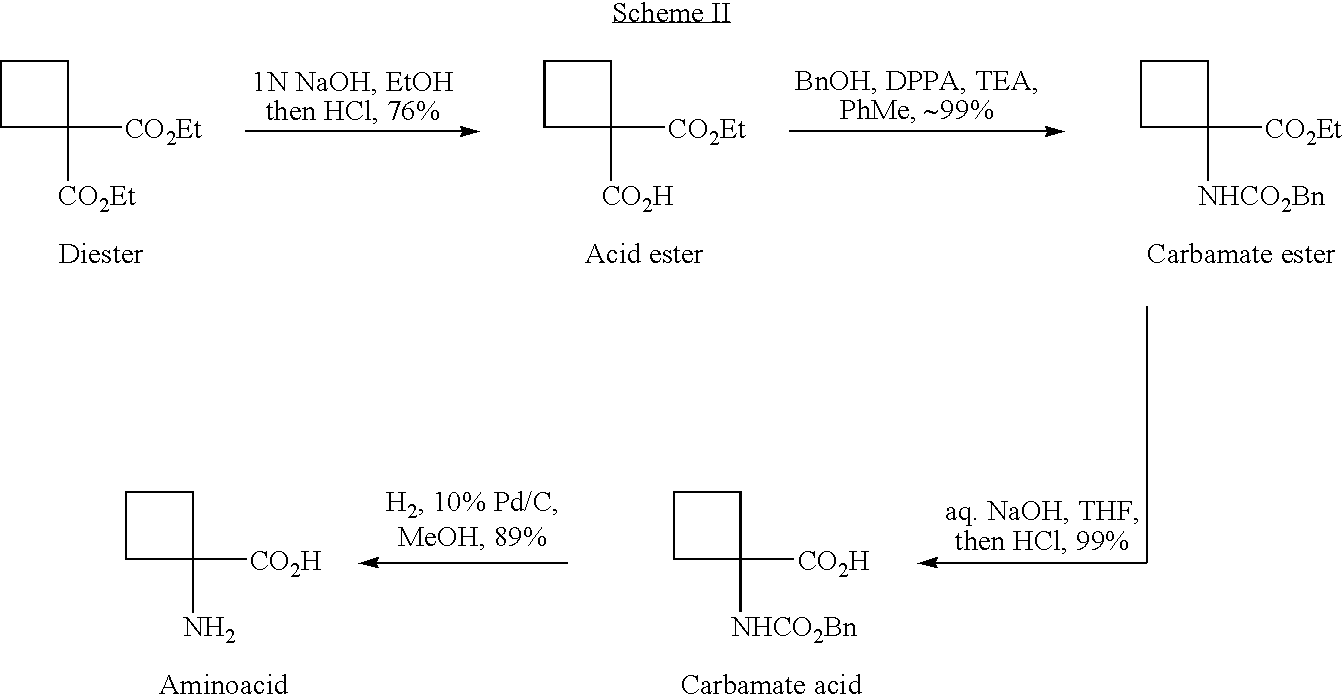
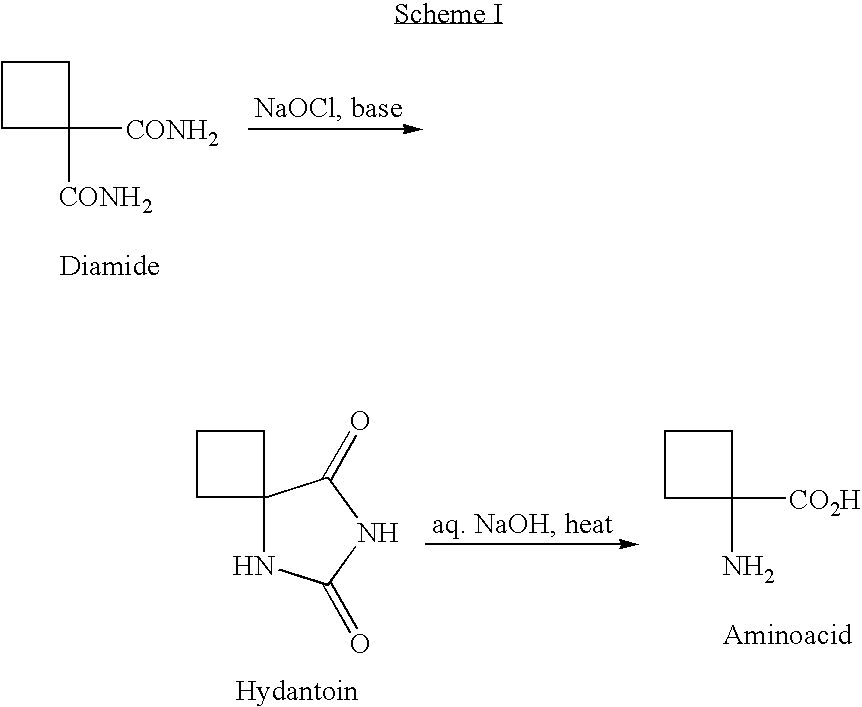
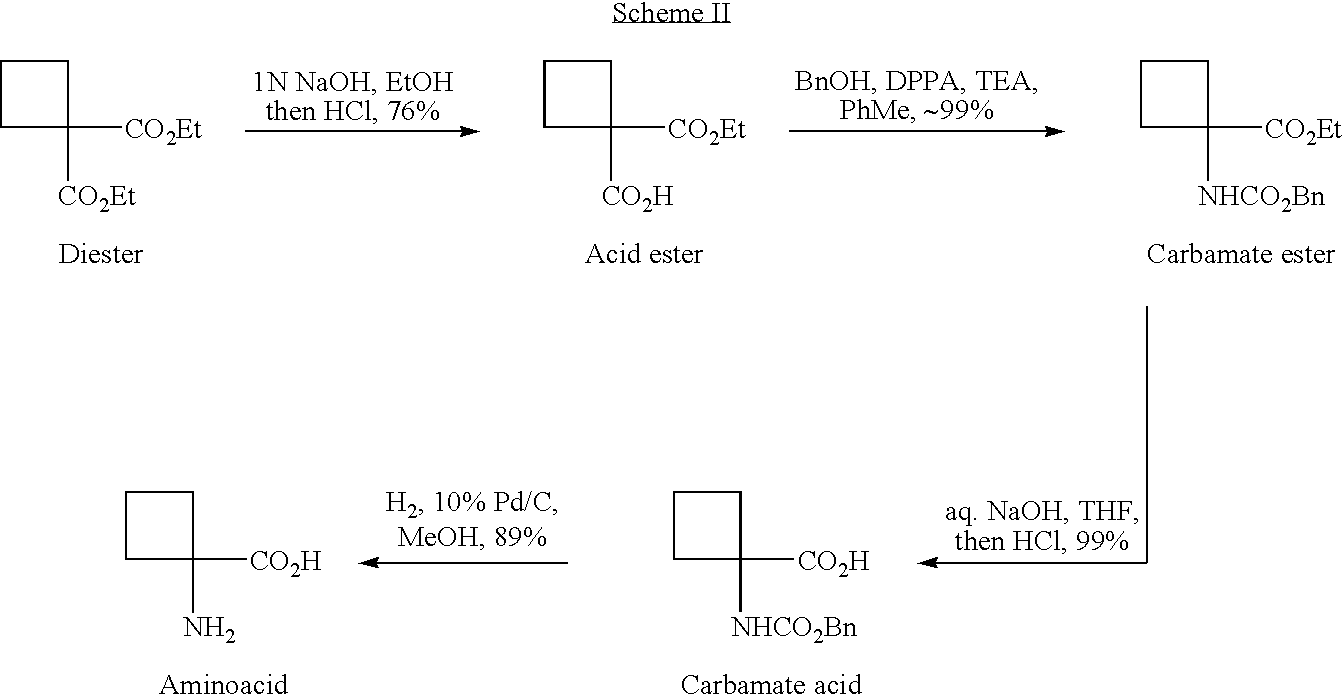
Cycloalkylamidoacid compounds, processes for making and uses thereof
The invention relates to the field of pharmaceutics and more specifically to novel cycloalkylamidoacid compositions useful in the preparation of cycloalkyaminoacids and oxazolidinediones, and processes for making cycloamidoacids. X and R are defined herein
Owner:BOEHRINGER INGELHEIM INT GMBH
Heterocyclic analogs of diphenylethylene compounds
Novel diphenylethylene compounds and derivatives thereof containing thiazolidinedione or oxazolidinedione moieties are provided which are effective in lowering blood glucose level, serum insulin, triglyceride and free fatty acid levels in animal models of Type II diabetes. The compounds are disclosed as useful for a variety of treatments including the treatment of inflammation, inflammatory and immunological diseases, insulin resistance, hyperlipidemia, coronary artery disease, cancer and multiple sclerosis.
Owner:THERAKOS INC
Antidiabetic Oxazolidinediones and Thiazolidinediones
InactiveUS20090069385A1Effective in lowering glucoseEffective in lipidBiocideOrganic chemistryAcute hyperglycaemiaDyslipidemia
Pyridinyloxyphenyl and pyridinyloxybenzyl oxazolidine-2,4-diones and thiazolidine-2,4-diones are agonists or partial agonists of PPAR gamma and are useful in the treatment and control of hyperglycemia that is symptomatic of type II diabetes, as well as dyslipidemia, hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, and obesity that are often associated with type 2 diabetes.
Owner:MERCK SHARP & DOHME CORP
Antidiabetic oxazolidinediones and thiazolidinediones
Phenoxyphenyl and phenoxybenzyl oxazolidine-2,4-diones and thiazolidine-2,4-diones are agonists or partial agonists of PPAR gamma and are useful in the treatment and control of hyperglycemia that is symptomatic of type II diabetes, as well as dyslipidemia, hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, and obesity that are often associated with type 2 diabetes.
Owner:MERCK SHARP & DOHME CORP
Cycloalkylamidoacid compounds, processes for making and uses thereof
The invention relates to the field of pharmaceutics and more specifically to cycloalkylamidoacid compositions useful in the preparation of cycloalkyaminoacids and oxazolidinediones, and processes for making cycloamidoacidsX and R are defined herein.
Owner:BOEHRINGER INGELHEIM INT GMBH
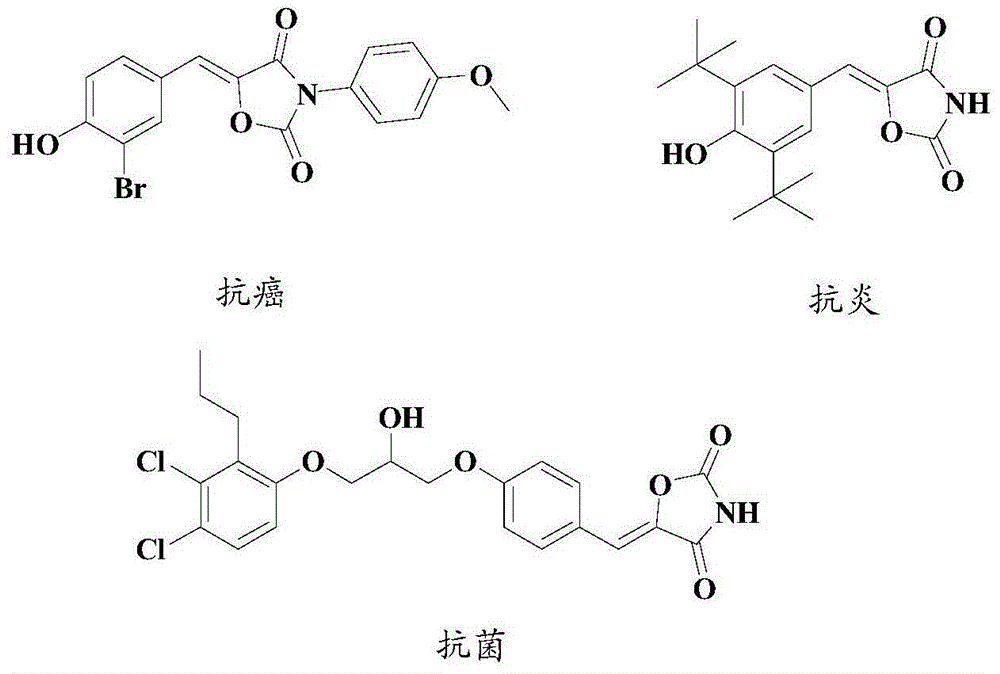
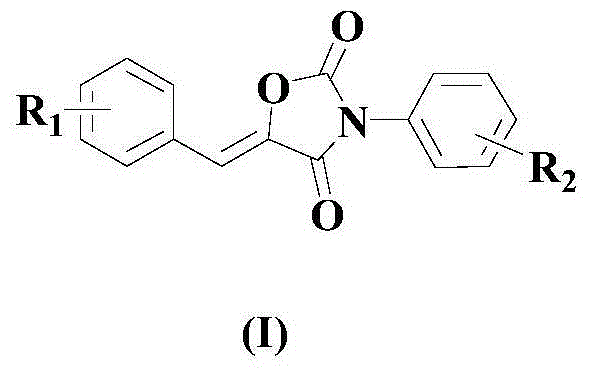
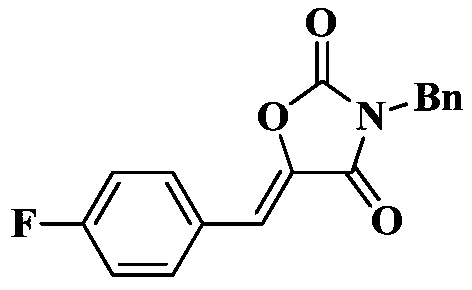
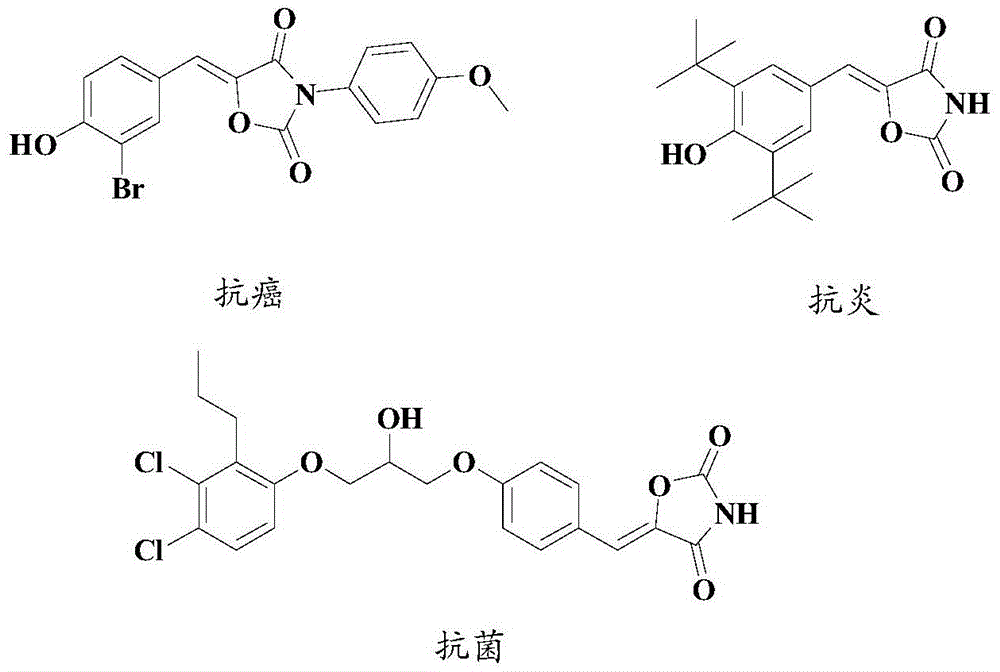
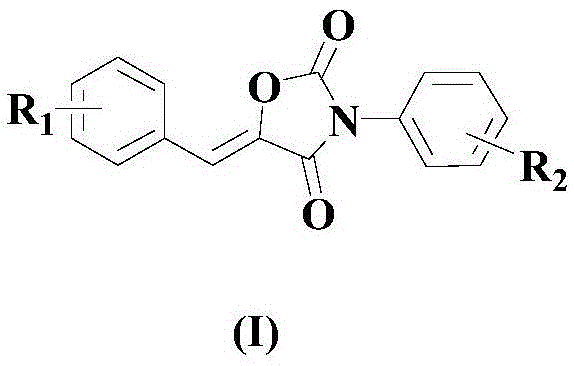
Synthesis method of drug intermediate oxazolidinedione compound
InactiveCN104672161AHigh yieldBroad application prospectsOrganic chemistryOxazolidinedioneAlkoxy group
The invention provides a synthesis method of an oxazolidinedione compound represented by formula (I) shown in the specification. The method comprises steps as follows: a compound represented by formula (II) shown in the specification, a compound represented by formula (III) shown in the specification and a compound represented by formula (IV) shown in the specification have a reaction in a solvent in the carbon dioxide atmosphere in the presence of a catalyst, a base and an aid, so that the oxazolidinedione compound represented by formula (I) is obtained; R1 and R2 are H, C1-C6 alkyl groups, C1-C6 alkoxy groups or halogens respectively and independently. According to the method, the good technical effect is realized through combination and cooperation of proper catalyst, base, aid and solvent systems, and the method has very broad market application prospect in the field of drug intermediate synthesis.
Owner:北京民康百草医药科技有限公司
Fungicidal mixtures
The present invention discloses an advantageous combination of an oxazolidinedione of formula (I) and cymoxanil (or an agriculturally suitable salt thereof) and its use in preventing and controlling plant fungal diseases.
Owner:EI DU PONT DE NEMOURS & CO
Method for producing tetra-substituted vinyl 2,4-oxazolidinedione from carbon dioxide
ActiveCN110590691AWide applicabilityHigh yieldOrganic chemistryChemical recyclingOxazolidinedioneHalohydrocarbon
The invention belongs to the technical field of fixation and conversion of carbon dioxide, particularly relates to a functionalization reaction of an alkynyl-containing compound, and discloses a method for producing tetra-substituted vinyl 2,4-oxazolidinedione from carbon dioxide. The method for producing the tetra-substituted vinyl 2,4-oxazolidinedione from the carbon dioxide comprises the specific technology steps: in a Schlenk tube subjected to dehydration and deoxidation, adding a catalyst, a copper salt, an alkali, propargylamide, halohydrocarbon, the carbon dioxide and a solvent according to a ratio, placing the Schlenk tube in an environment at environmental temperature, and conducting stirring for 16-24 hours; and finally, conducting silica gelcolumn chromatography to obtain the tetra-substituted vinyl 2,4-oxazolidinedione. According to the method for producing the tetra-substituted vinyl 2,4-oxazolidinedione from the carbon dioxide, the CO2 is adopted as a carboxyl source forthe first time, and at the environmental temperature, the tetra-substituted vinyl 2,4-oxazolidinedione is built.
Owner:CHANGZHOU UNIV
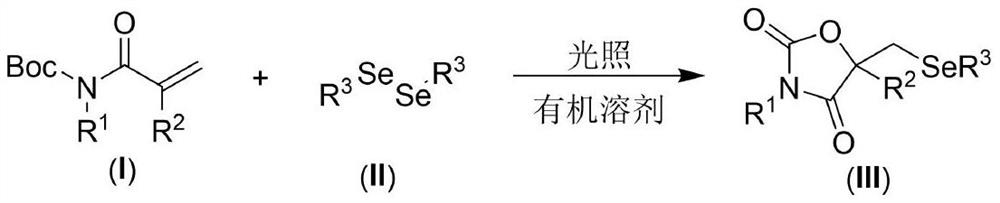
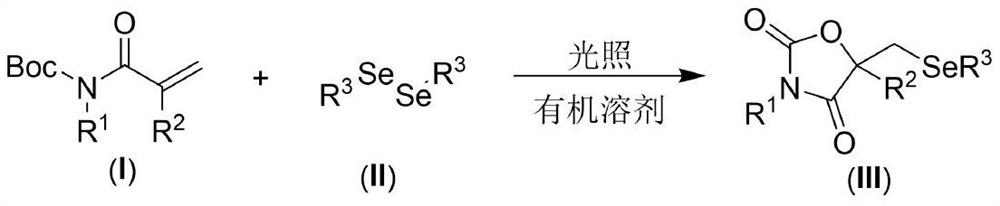
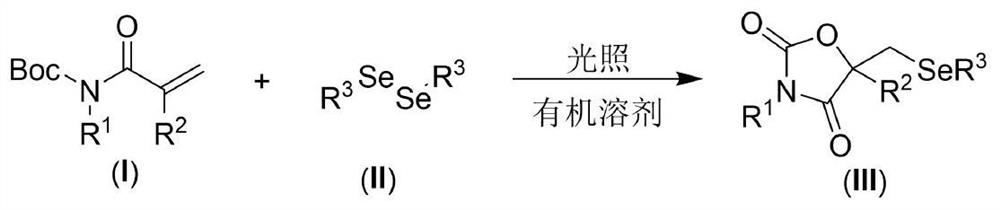
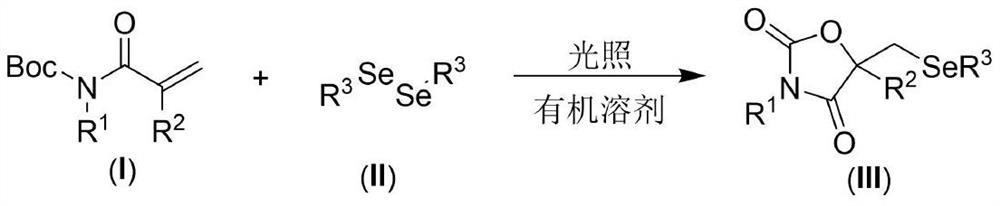
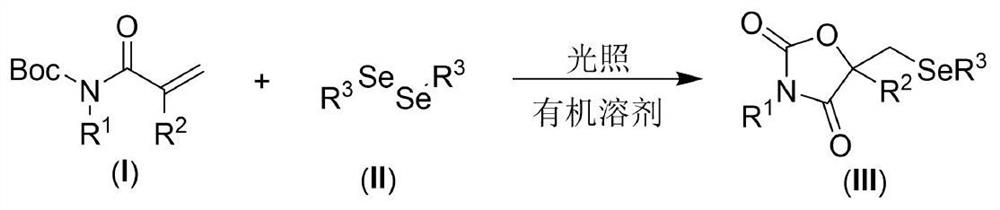
Visible light promoted synthesis method of seleno oxazolidine-2. 4-diketone
The invention discloses a visible light promoted synthesis method of seleno-oxazolidine-2, 4-diketone, the method comprises the following steps: taking N-Boc protected acrylamide and diselenide as reaction raw materials, stirring and reacting at a certain temperature under the irradiation of a light source to obtain a seleno-oxazolidine-2, 4-diketone compound. According to the method, the reaction conditions are simple, the yield of the product is high, and the preparation from the acrylamide raw material to the seleno-oxazolidine-2, 4-diketone heterocyclic compound is realized for the first time; the method also has the advantages of being insensitive to air, wide in substrate range and the like, develops a new synthesis route and method for the seleno-oxazolidine-2, 4-diketone heterocyclic compound, and has good application potential and research value.
Owner:NANTONG UNIVERSITY
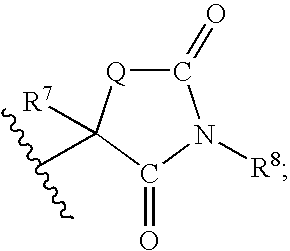
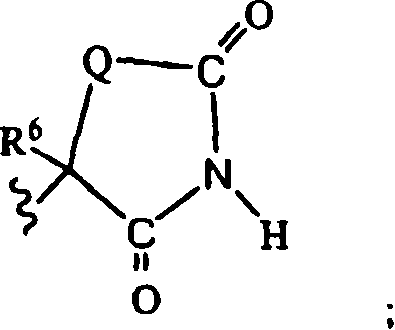
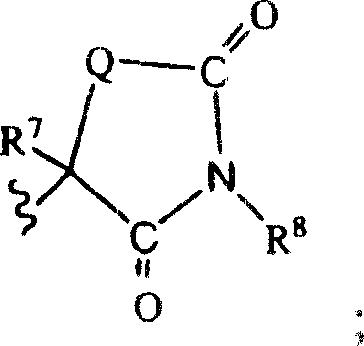
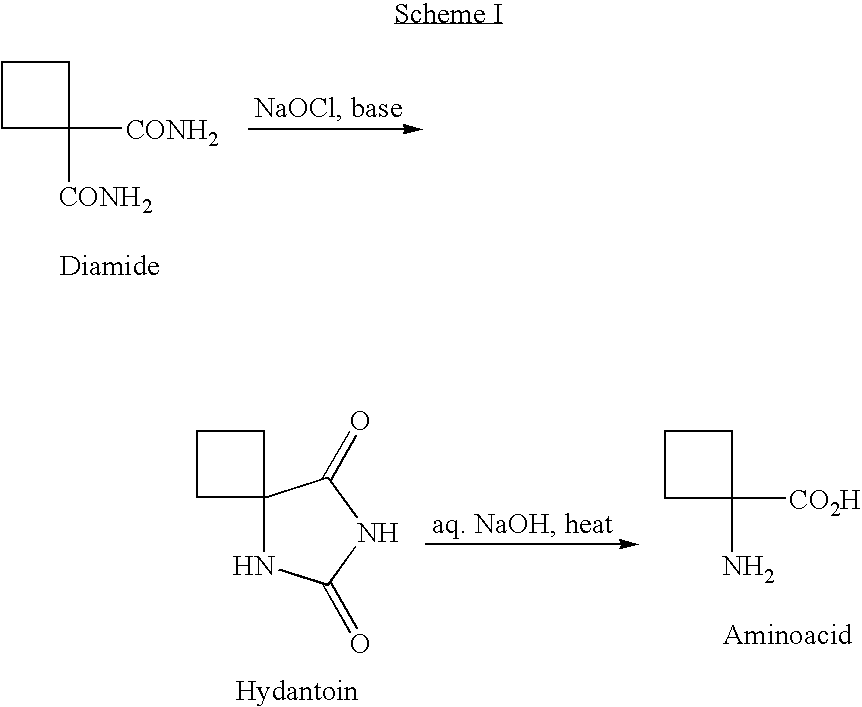
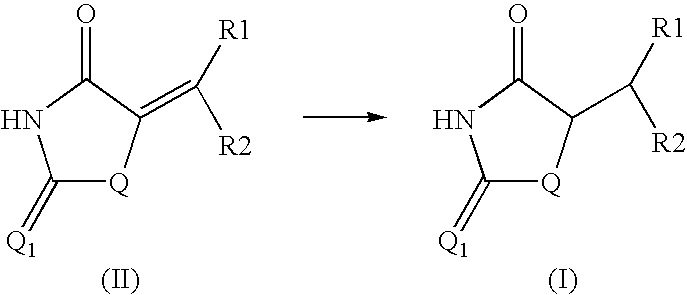
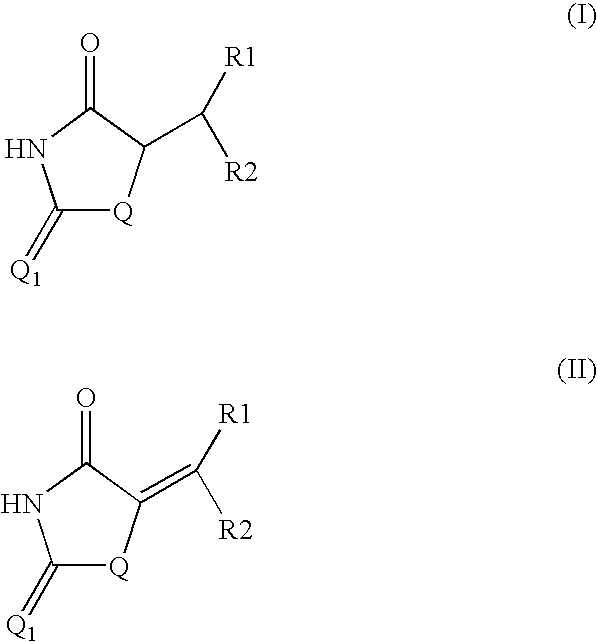
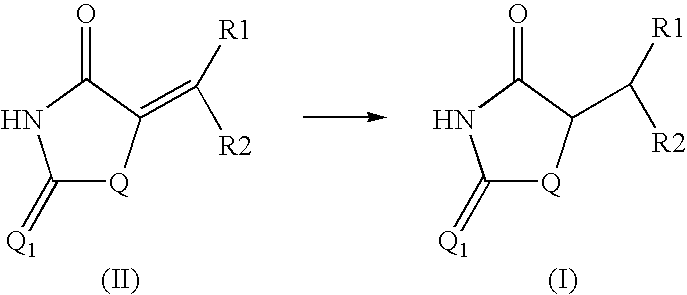
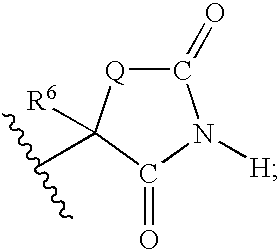
Method for preparing compounds derived from thiazolidinedione, oxazolidinedione or hydantoin
A method for preparing a thiazolidinedione, oxazolidinedione or hydantoin compound of formula (I) from a compound of formula (II): wherein Q represents an oxygen atom or a sulfur atom; Q1 represents an oxygen atom or a sulfur atom; R1 and R2, which can be identical or different, represent a hydrogen atom, a C1-10 alkyl chain, a cycloalkyl, an alkylaryl, an arylalkyl; the alkyl, cycloalkyl, alkylaryl or arylalkyl groups being optionally substituted by an alkyl, an alkoxy or aryloxy, a halogen, a hydroxy, a sulfino, a sulfonyl, an amino such as NH2, NHR3, N(R3)2, wherein R3 represents an alkyl, an alkoxy or an alkylcarbonyl, reacting a compound of formula (II) with formic acid, either as a hydrogen donor in a hydrogen-transfer reaction or as a solvent in a hydrogenation reaction, in the presence of a catalyst containing a transition metal to obtain a corresponding compound of formula (I).
Owner:PPG SIPSY
Antidiabetic Oxazolidinediones and Thiazolidinediones
InactiveUS20100168164A1Effective in lowering glucoseEffective in lipidBiocideOrganic chemistryDyslipidemiaOxazolidinedione
Phenoxyphenyl and phenoxybenzyl oxazolidine-2,4-diones and thiazolidine-2,4-diones are agonists or partial agonists of PPAR gamma and are useful in the treatment and control of hyperglycemia that is symptomatic of type II diabetes, as well as dyslipidemia, hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, and obesity that are often associated with type 2 diabetes.
Owner:MERCK SHARP & DOHME CORP
Resist composition and pattern forming process
ActiveUS9720324B2High resolutionSuppress DiffusePhotomechanical coating apparatusPhotomechanical exposure apparatusOxazolidinedionePolymer science
A resist composition is provided comprising a polymer comprising recurring units (a) having an oxazolidinedione, thioxooxazolidinone, thiazolidinedione or thioxothiazolidinone structure and recurring unit (b1) having an acid labile group-substituted carboxyl group and / or recurring units (b2) having an acid labile group-substituted phenolic hydroxyl group. The resist composition suppresses acid diffusion, exhibits a high resolution, and forms a pattern of satisfactory profile with low edge roughness.
Owner:SHIN ETSU CHEM IND CO LTD
An organic amine catalyzed co 2 Method for synthesizing 2,4-oxazolidinediones
The invention provides a method used for synthesizing 2, 4-oxazolidinedione compound through organic amine catalyzing CO2, and belongs to the technical field of organic synthesis, pesticide, and medical chemistry. The method comprises following steps: acetylene amide raw material and a solvent are introduced into a reaction bottle containing a magneton, an organic base is added as a catalyst, thereaction bottle is introduced into a Schlink bottle filled with carbon dioxide gas, stirring reaction is carried out for 1 to 6h at room temperature, after reaction, an obtained reaction liquid is pumped out from the reaction bottle, an obtained crude product is subjected to column chromatography so as to obtain the 2, 4-oxazolidinedione compound. According to the method, the simple organic base of a catalytic amount is adopted to replace a conventional metal catalyst; reaction is clean; reaction conditions are mild; function group tolerance is high; conversion rate and stereoselectivity are high, and the application prospect in the fields of organic synthesis, pesticide, and medicine is promising.
Owner:DALIAN UNIV OF TECH
A kind of synthetic method of pharmaceutical intermediate oxazolidinedione compound
The invention provides a synthesis method of an oxazolidinedione compound represented by formula (I) shown in the specification. The method comprises steps as follows: a compound represented by formula (II) shown in the specification, a compound represented by formula (III) shown in the specification and a compound represented by formula (IV) shown in the specification have a reaction in a solvent in the carbon dioxide atmosphere in the presence of a catalyst, a base and an aid, so that the oxazolidinedione compound represented by formula (I) is obtained; R1 and R2 are H, C1-C6 alkyl groups, C1-C6 alkoxy groups or halogens respectively and independently. According to the method, the good technical effect is realized through combination and cooperation of proper catalyst, base, aid and solvent systems, and the method has very broad market application prospect in the field of drug intermediate synthesis.
Owner:北京民康百草医药科技有限公司
Benzoureas Having Anti-Diabetic Activity
InactiveUS20080076810A1Efficacious in treatmentUseful in controlBiocideOrganic chemistryDyslipidemiaOxazolidinedione
Benzourea compounds of Formula I having aryl-(CH2)x-oxazolidinedione or aryl-(CH2)x-thiazolidinedione substituents on one of the N atoms of the benzourea ring, wherein x is 0 or 1, are PPAR gamma agonists or partial agonists and are useful in the treatment and control of type II diabetes, including hyperglycemia and other symptoms such as dyslipidemia, hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, and obesity, that are often associated with type 2 diabetes.
Owner:MERCK SHARP & DOHME CORP
A visible light-promoted synthesis method of selenooxazolidine-2,4-dione
The invention discloses a synthesis method of selenooxazolidine-2.4-diketone promoted by visible light, using N-Boc-protected acrylamide and diselenide as reaction raw materials, and performing a stirring reaction under certain temperature conditions and light source irradiation , to obtain selenooxazolidine-2,4-dione compound. The reaction condition of the method is simple, the yield of the product is high, and the preparation of the selenooxazolidine-2,4-diketone heterocyclic compound from the raw material of acrylamide is realized for the first time; It has the advantages of wide range, opens up a new synthetic route and method for selenooxazolidine-2,4-diketone heterocyclic compounds, and has good application potential and research value.
Owner:NANTONG UNIVERSITY
Method for synthesizing 3-(2-phenylethyl)-5-[2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolin-en]-2,4-oxazolidinedione
InactiveCN101121714BSimple preparation processRaw materials are cheap and easy to getOrganic chemistryOxazolidinedioneEthyl group
The invention discloses a synthesis method of the 3-(2-phenyl ethyl)-5-[2, 3, 6, 7-tetrahydrogen-1H, 5H-dibenzo [ij] quinoline-9-ene]-2, 4-oxazolidine dione; the steps first uses the hydroxy ethyl acetate and urea as the raw materials to make the oxazolidine-2 ,4-dione; then the oxazolidine-2 ,4-dione reacts with the beta-bromophenyl ethane to make the 3-phenethyl-oxazolidine-2, 4-dione; the 3-phenethyl-oxazolidine-2, 4-dione then reacts with the 2,3,6,7-tetrahydrogen-1 H, 5H-pyrido [3,2,1-ij] quinoline-9-formaldehyde to make the product 3-(2-phenyl ethyl)-5-[2,3,6, 7-tetrahydrogen-1 H, 5H-benzo [ij] quinoline-9-ene]-2,4-oxazolidine dione. The invention is of the simple process, the easily accessible and cheap raw materials, the high production rate, the low cost and the suitableness forthe industrial production.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
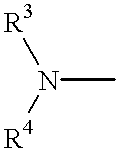
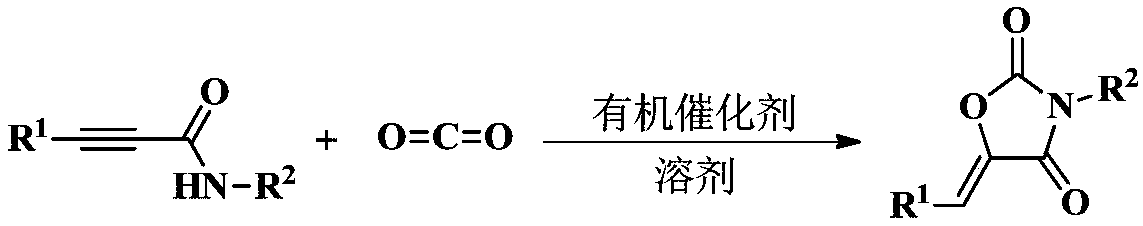
![Method for synthesizing 3-(2-phenylethyl)-5-[2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolin-en]-2,4-oxazolidinedione Method for synthesizing 3-(2-phenylethyl)-5-[2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolin-en]-2,4-oxazolidinedione](https://images-eureka.patsnap.com/patent_img/ed840db5-a882-497a-8224-59829809f46f/A20071007060500031.PNG)
![Method for synthesizing 3-(2-phenylethyl)-5-[2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolin-en]-2,4-oxazolidinedione Method for synthesizing 3-(2-phenylethyl)-5-[2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolin-en]-2,4-oxazolidinedione](https://images-eureka.patsnap.com/patent_img/ed840db5-a882-497a-8224-59829809f46f/A20071007060500041.PNG)


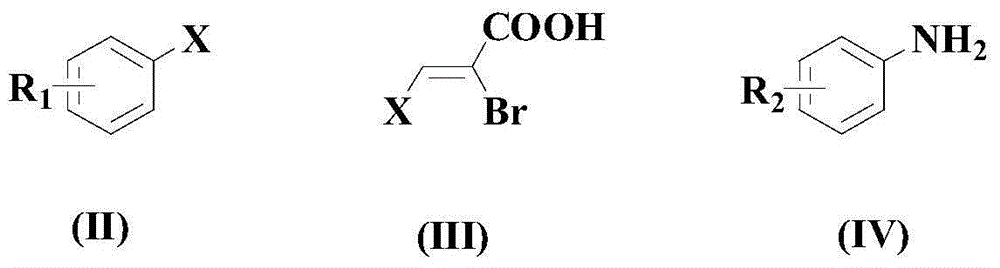
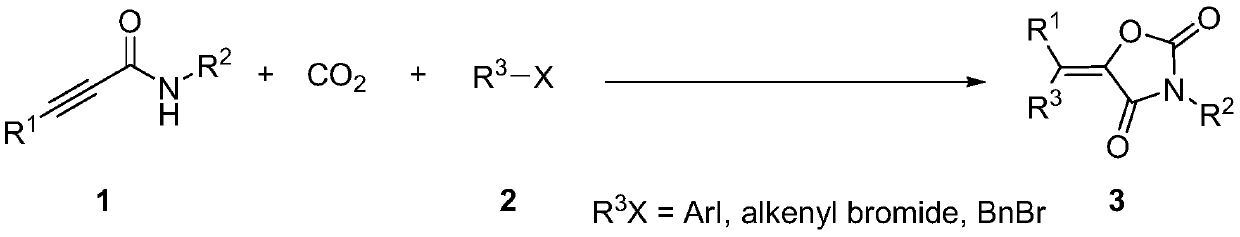

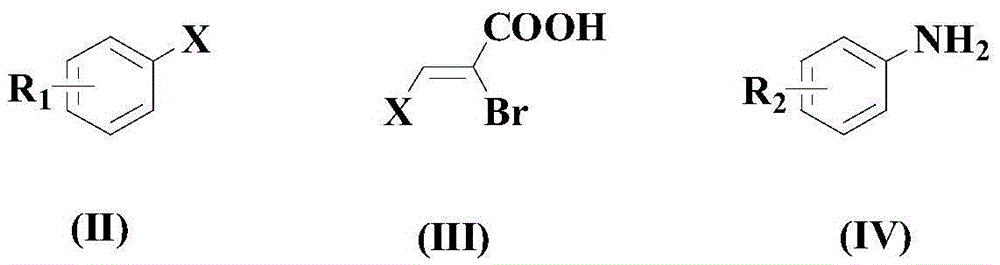
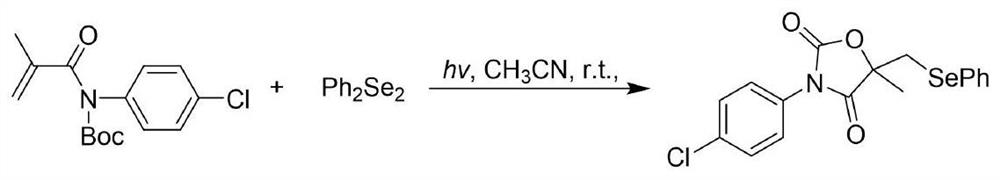
![Method for synthesizing 3-(2-phenylethyl)-5-[2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolin-en]-2,4-oxazolidinedione Method for synthesizing 3-(2-phenylethyl)-5-[2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolin-en]-2,4-oxazolidinedione](https://images-eureka.patsnap.com/patent_img/fc991820-3e43-4649-a0c6-b66cb053dc3b/G07170605820070925D000011.PNG)
![Method for synthesizing 3-(2-phenylethyl)-5-[2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolin-en]-2,4-oxazolidinedione Method for synthesizing 3-(2-phenylethyl)-5-[2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolin-en]-2,4-oxazolidinedione](https://images-eureka.patsnap.com/patent_img/fc991820-3e43-4649-a0c6-b66cb053dc3b/G07170605820070925D000021.PNG)