Method for directly synthesizing oxazolidin-2,4-one heterocyclic compound from alkyne amide
A technology of heterocyclic compounds and alkyne amides, which is applied in the field of organic chemical synthesis, can solve the problems of difficult electrolysis, cumbersome synthesis steps, and long reaction time, and achieve the effects of high yield, high reaction efficiency, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
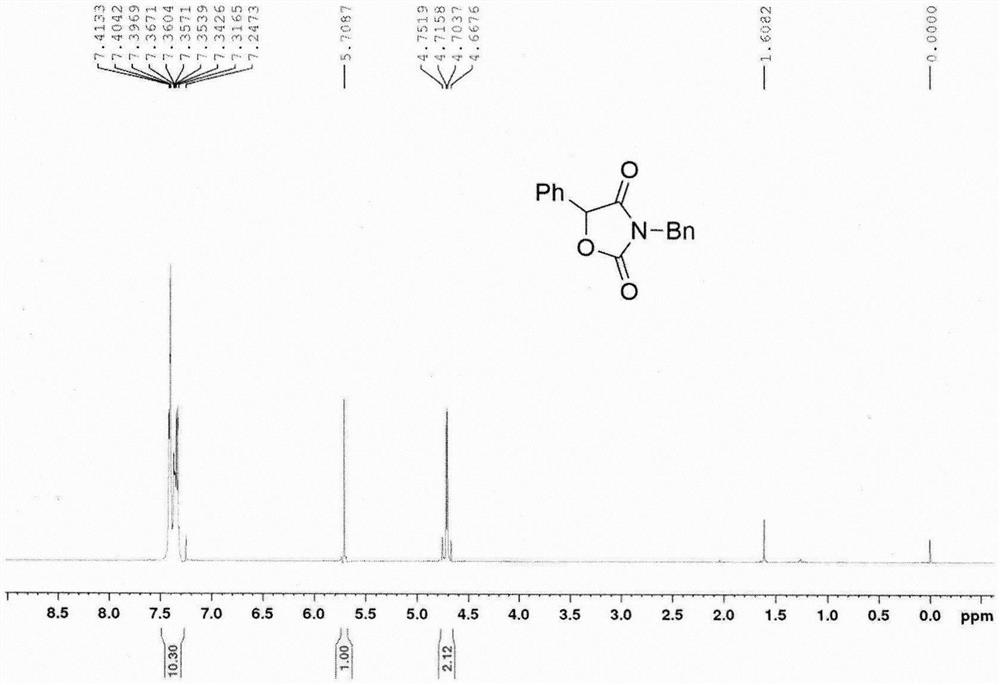
[0018] In air, 1.8425 g of N-phenylethynyl-N-benzylcarbamate tert-butyl ester, 0.2124 g of 1-chloromethyl-4-fluoro-1,4-azonium bicyclo[2.2.2] Octane bistetrafluoroborate (Selectfluor TM ) into a 100mL reaction flask, 60mL dimethyl sulfoxide was added, and placed in an oil bath at 100°C to react for 1.5 hours. Cool the reaction solution to room temperature, add 20 mL of water, then extract three times with 60 mL of ethyl acetate, combine the organic phases, wash with 30 mL of saturated brine, dry over anhydrous sodium sulfate, filter, distill off excess solvent under reduced pressure, and perform column chromatography (Petroleum ether:ethyl acetate=10:1) to obtain 1.4821 g of 3-phenyl-5-benzyloxazolidine-2,4-dione. Melting point 114.1-114.9°C, 1 H NMR (400MHz, CDCl 3 )δ7.41-7.32 (m, 10H), 5.71 (s, 1H), 4.75-4.67 (m, 2H); 13 C NMR (100MHz, CDCl 3 )δ170.9, 155.1, 134.6, 131.6, 129.8, 129.1, 128.9, 128.8, 128.6, 126.1, 80.2, 44.0.
Embodiment 2
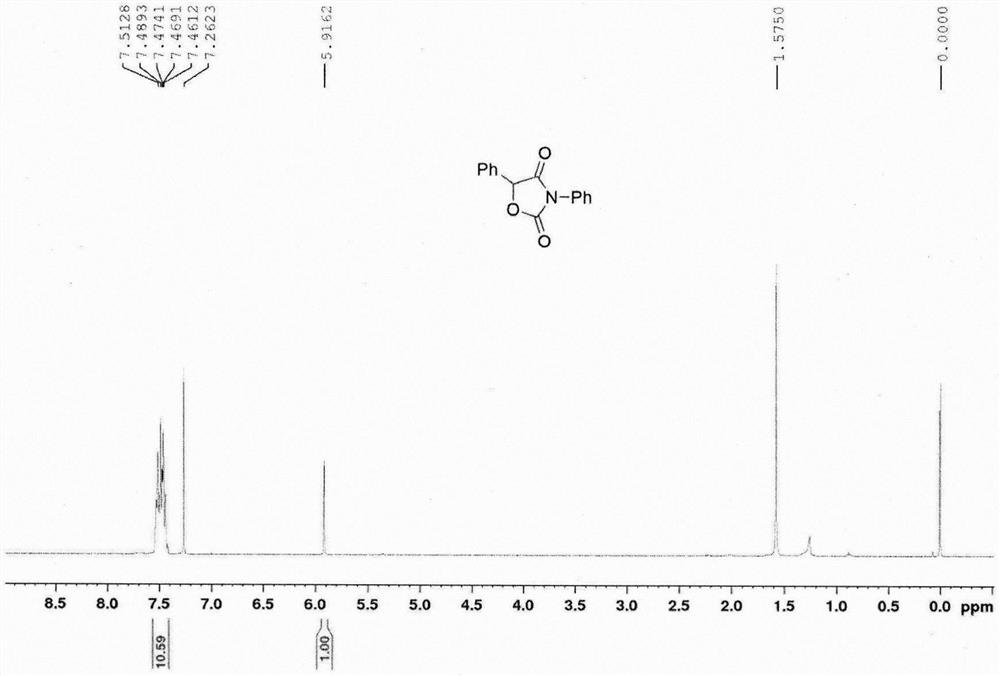
[0020] In air, successively add 1.7581 g of tert-butyl N-phenylethynyl-N-phenylcarbamate, 0.2124 g of 1-chloromethyl-4-fluoro-1,4-azoniumbicyclo[2.2.2]octane Alkane bistetrafluoroborate (Selectfluor TM ) into a 100 mL reaction flask, 60 mL of dimethyl sulfoxide was added, and the reaction was carried out in an oil bath at 100°C for 1 hour. Cool the reaction solution to room temperature, add 20 mL of water, then extract three times with 60 mL of ethyl acetate, combine the organic phases, wash with 30 mL of saturated brine, dry over anhydrous sodium sulfate, filter, distill off excess solvent under reduced pressure, and use petroleum ether Recrystallization from ethyl acetate gave 1.3983 g of 3,5-diphenyloxazolidine-2,4-dione. Melting point 118.7-120.4°C, 1 H NMR (400MHz, CDCl 3 )δ7.26-7.51(m, 10H), 5.92(s, 1H). 13 C NMR (100MHz, CDCl 3 )δ170.0, 154.0, 131.5, 130.7, 130.0, 129.4, 129.2, 129.1, 126.08, 125.6, 79.9.
Embodiment 3
[0022] In air, sequentially add 0.2571 g of tert-butyl N-phenylethynyl-N-allyl carbamate, 0.0176 g of 50% tetrafluoroboric acid aqueous solution into a 20 mL reaction tube, add 15 mL of N,N-dimethyl Formamide was placed in an oil bath at 120°C for 3 hours. Cool the reaction solution to room temperature, add 10 mL of water, then extract three times with 20 mL of ethyl acetate, combine the organic phases, wash with 20 mL of 5% dilute hydrochloric acid, 30 mL of saturated saline, dry over anhydrous magnesium sulfate, filter, and distill off excess under reduced pressure. solvent, column chromatography (petroleum ether: ethyl acetate = 10:1) to obtain 0.1945 g of 3-allyl-5-phenyloxazolidine-2,4-dione. Melting point 60.1-61.5°C, 1 H NMR (400MHz, CDCl 3 )δ7.43 (m, 5H), 5.88-5.80 (m, 1H), 5.75 (s, 1H) 5.33-5.26 (t, J = 10.2Hz, 2H), 4.20-4.18 (m, 2H); 13 C NMR (100MHz, CDCl 3 )δ 170.7, 154.9, 131.5, 129.8, 129.6, 129.2, 126.0, 119.6, 80.2, 42.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com