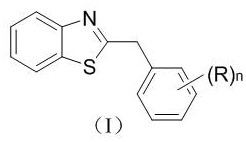
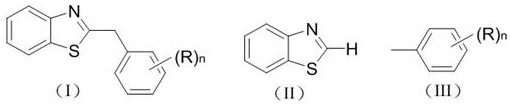
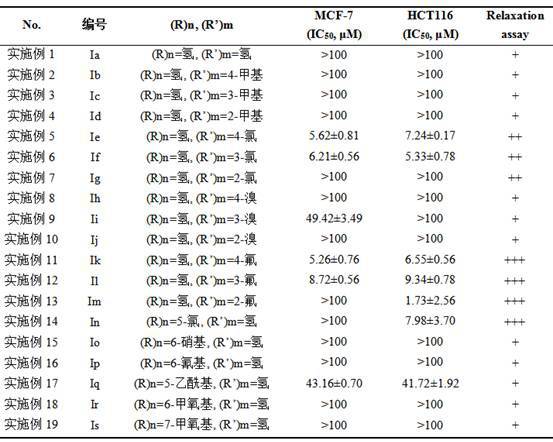
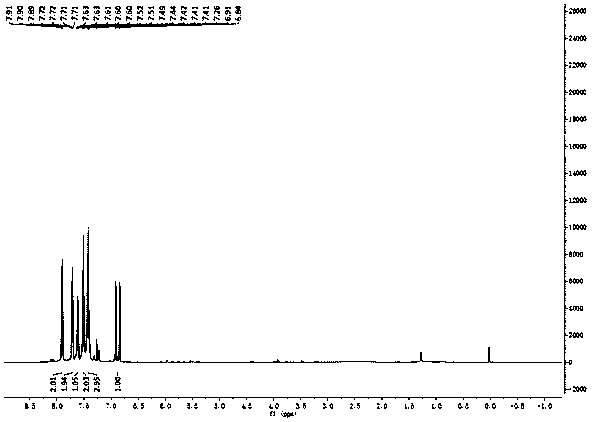
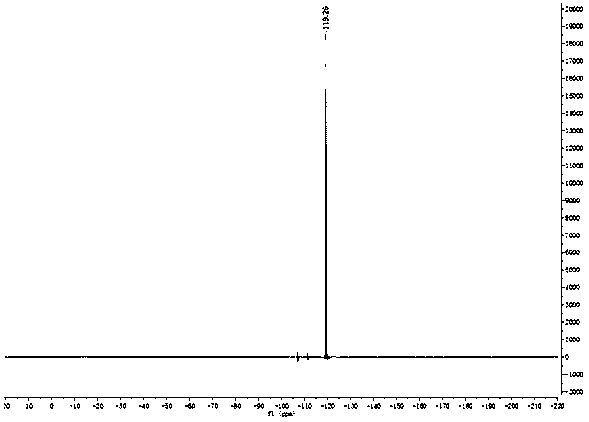
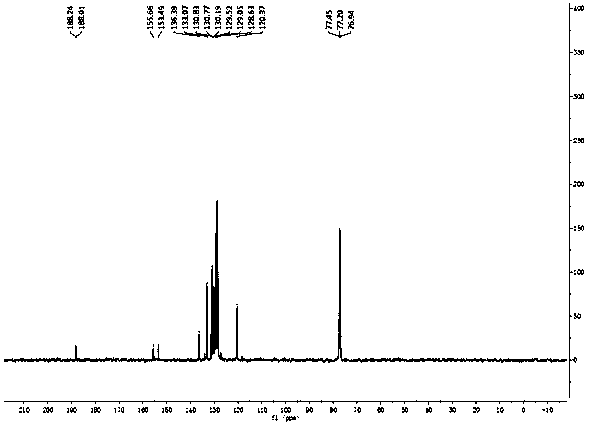
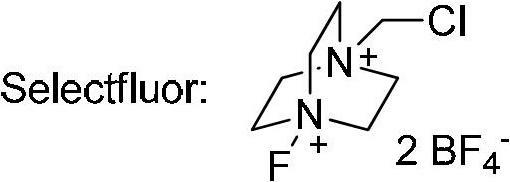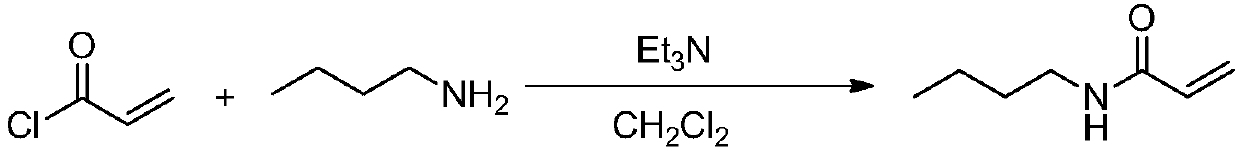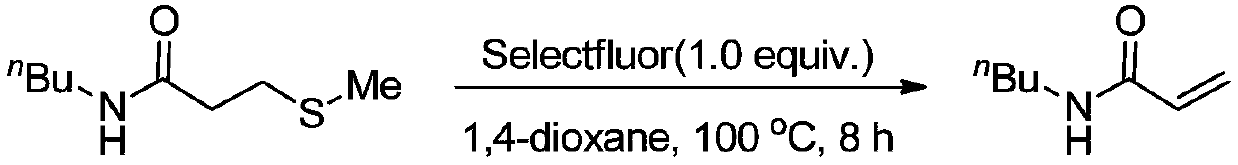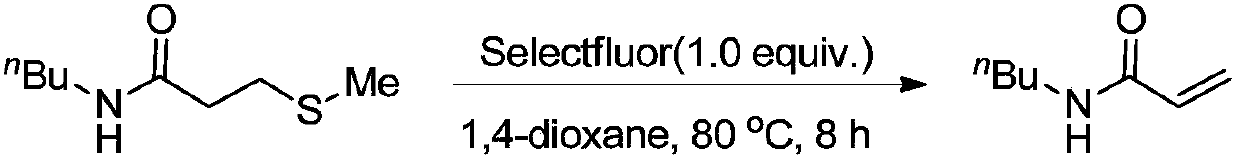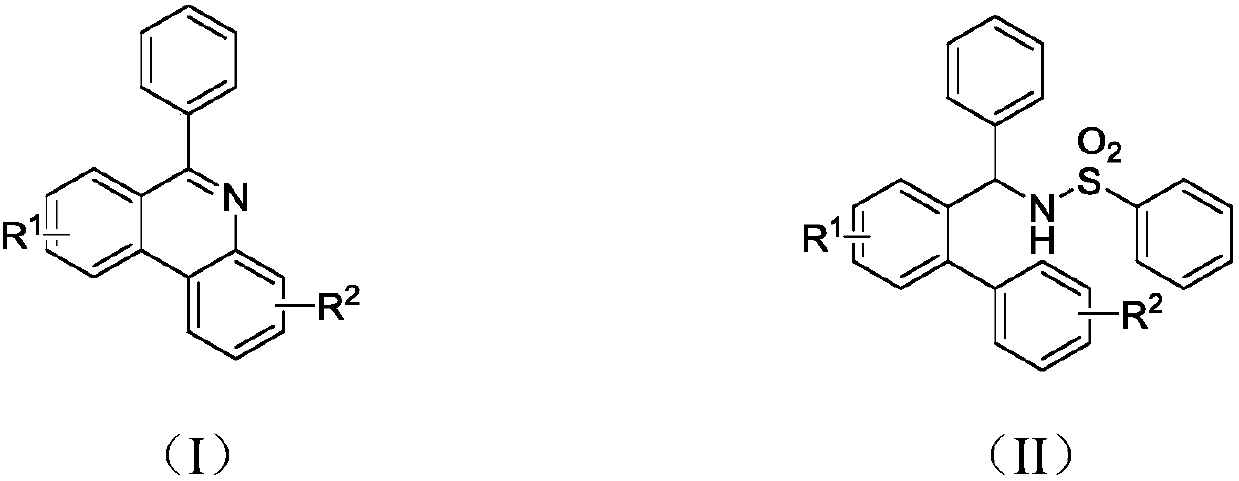Patents
Literature
86 results about "Selectfluor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) or Selectfluor, a trademark of Air Products and Chemicals, is a reagent in chemistry that is used as a fluorine donor. This compound is a derivative of the heterocycle DABCO . This colourless salt was first described in 1992 and has since been commercialized for use in organofluorine chemistry for electrophilic fluorination.
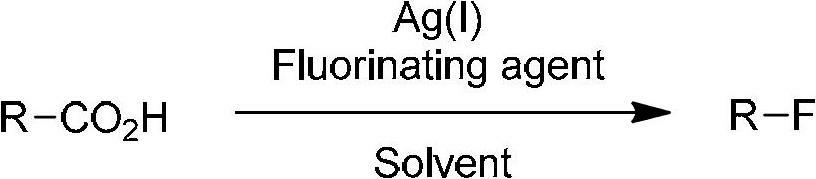
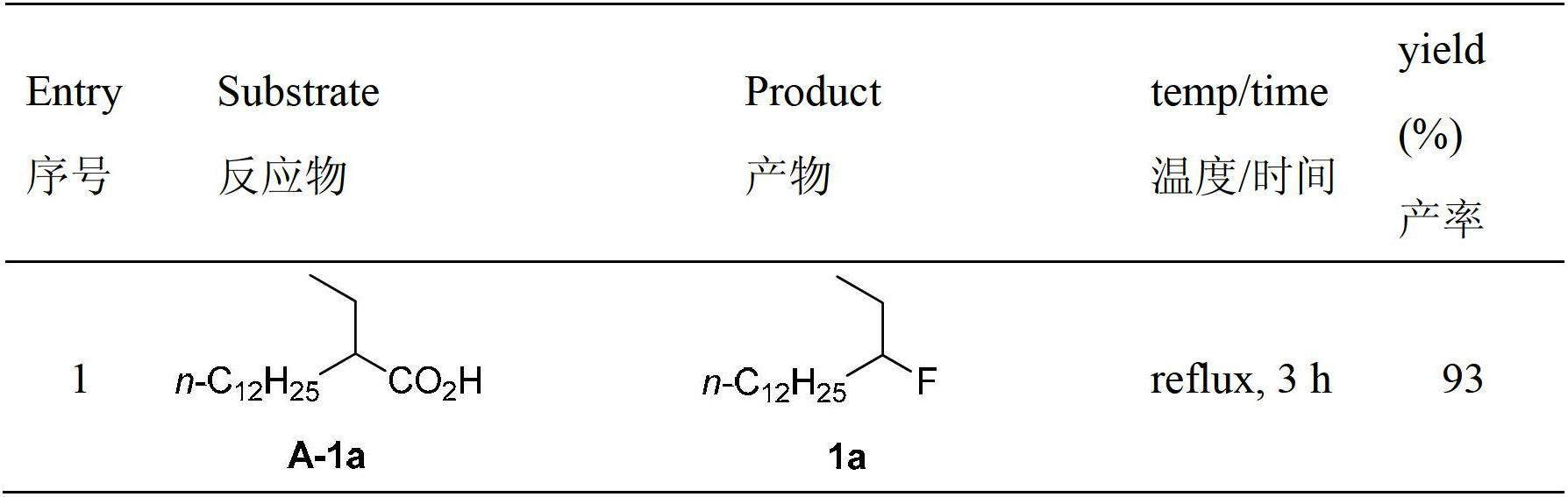
Decarboxylation and fluorination method for carboxylic acid
ActiveCN102675015AOrganic compound preparationCarboxylic acid esters preparationCarbon–fluorine bondRoom temperature
The invention relates to a decarboxylation and fluorination method for carboxylic acid. According to the method, fatty acid RCOOH, monovalent silver salt catalyst and fluorinated reagent Selectfluor or hexafluorophosphate derivative thereof are reacted in a solvent at the temperature of between room temperature and 80 DEG C to obtain RF; and by adopting the method, the carboxylic acid is efficiently converted into corresponding decarboxylated and fluorinated product in an aqueous phase. The method is mild in reaction conditions and easy to operate, has good chemical selectivity and functional group compatibility, and is a very practical sp<3> carbon-fluorine bond synthesizing method.
Owner:上海睿瓦科技有限公司
Preparation method of sulfuryl fluoride compound
InactiveCN105198683AHigh yieldMild reaction conditionsSulfonyl/sulfinyl group formation/introductionSulfonic acid preparationChemical synthesisSulfohydrazide
The invention relates to a preparation method of a sulfuryl fluoride compound. A sulfonyl hydrazide compound and a fluoride reagent serve as reaction raw materials. The preparation method includes the steps that a, the sulfonyl hydrazide compound with the structure (I) and the fluoride reagent are dispersed in a solvent, wherein the structure (I) is shown in the specification; b; a mixture obtained from the step a is stirred and heated to obtain the sulfuryl fluoride compound with the structure (II), wherein the structure (II) is shown in the specification (II). Compared with existing related technologies in the chemical synthesis field, the method of preparing sulfuryl fluoride from sulfonyl hydrazide is achieved for the first time. In the method, no catalyst needs to be added, reaction conditions are moderate, good compatibility can be achieved for water and air, and large-scale production is easy to achieve. The experimental result indicates that the yield of the obtained sulfuryl fluoride compound can reach up to 98%.
Owner:XINYANG NORMAL UNIVERSITY
Synthesis method of 2-position aryl substituted benzofuran ring 3-position fluoro
InactiveCN105837539AEfficient conversionMild reaction conditionsOrganic chemistryTetrafluoroborateSynthesis methods
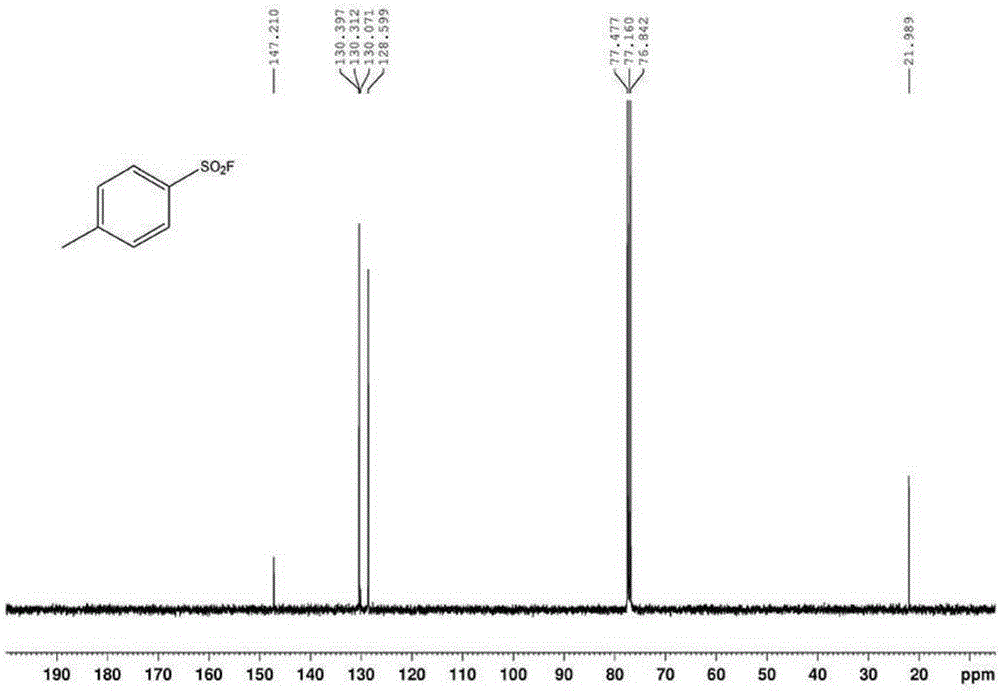
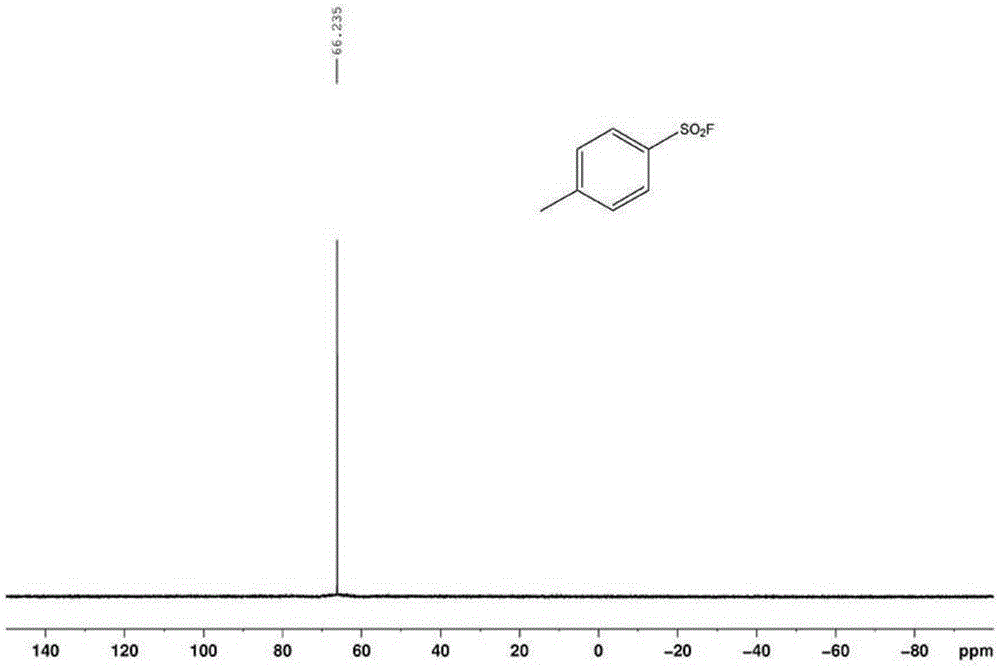
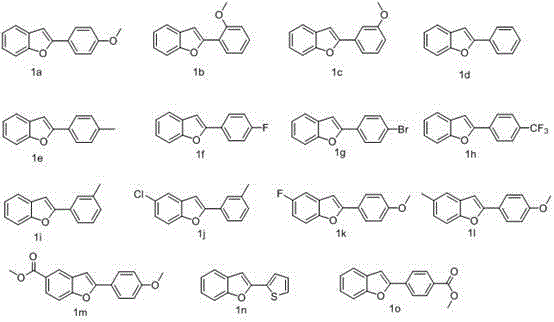
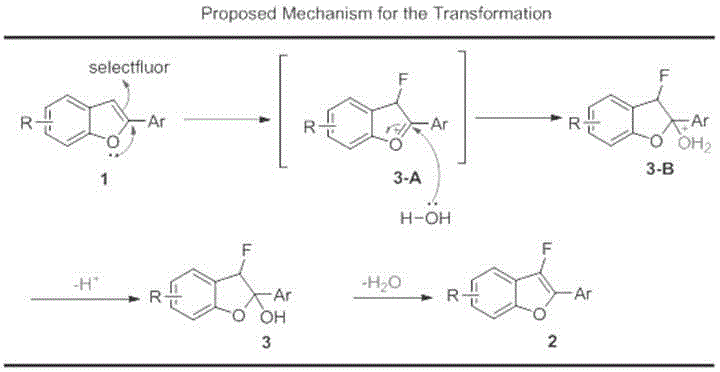
The invention relates to the technical field of fluorine organic compounds, in particular to a synthesis method of 2-position aryl substituted benzofuran ring 3-position fluoro. The method takes 1-chloromethyl-4-fluorodiazabicyclo[2, 2, 2]octane bis(tetrafluoroborate) (Selectfluor) as a fluorinating reagent, in a mixed solvent of acetonitrile and nucleophilic reagent water, a benzofuran compound is efficiently converted into corresponding 3-fluoro-2-ol-2, 3-dihydrobenzofuran, 3-fluoro-2-ol-2, 3-dihydrobenzofuran compound adopts pyridine and thionyl chloride as the dehydrating agent, and can be efficiently converted into a 3-position fluoro 2-position aryl substituted benzofuran compound. The method provided by the invention has the characteristics of mild reaction condition, easy operation, good chemical selectivity and functional group compatibility, and is an effective method for synthesis of 2-position aryl substituted benzofuran ring 3-position fluoro. (reaction formula).
Owner:FUDAN UNIV
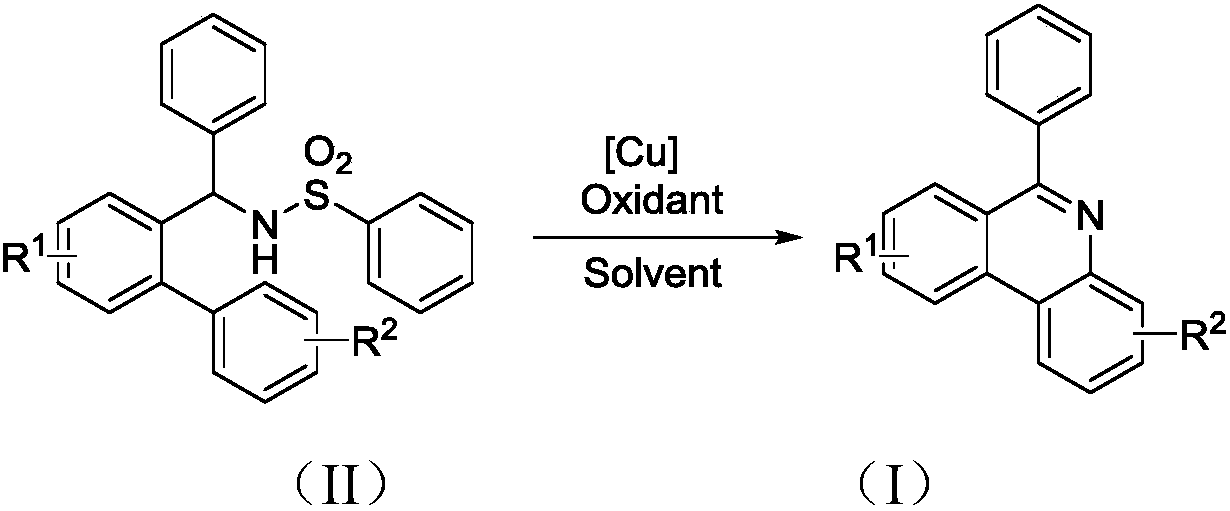
Synthesis method of phenanthridine and derivative of phenanthridine
InactiveCN107778239AReduce consumptionRaw materials are easy to obtainOrganic chemistryOrganic solventSynthesis methods
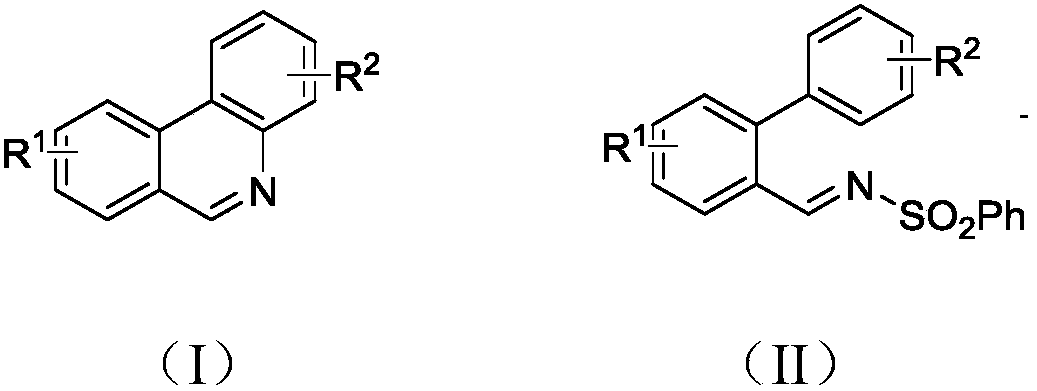
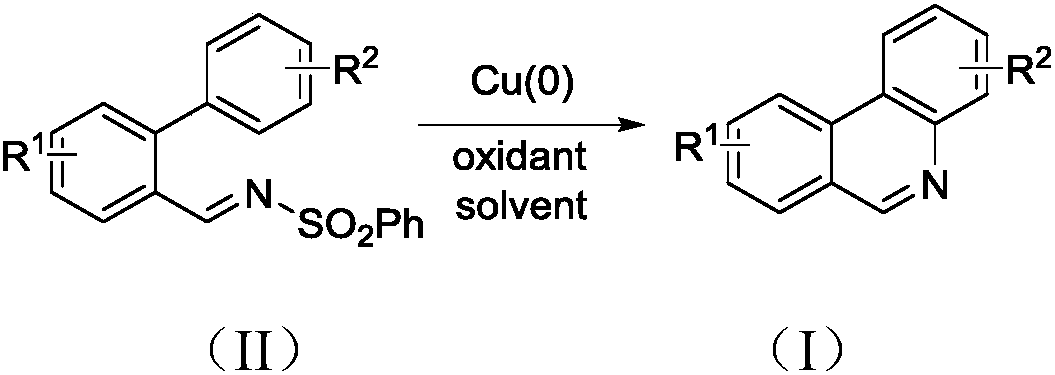
The invention discloses a synthesis method of phenanthridine as shown in a formula (I) and a derivative of the phenanthridine. In the synthesis method, o-arylphenylsulfimide shown as a formula (II) isused as a raw material and reacts in an organic solvent under the action of a [Cu] / Selectfluor catalyst to obtain a corresponding target product (I). The synthesis method disclosed by the invention has the advantages of cheap and easily available and low-toxicity catalyst, environmental friendliness, mild reaction conditions, high universality of functional group and easiness and convenience in operation. The formulas (I) and (II) are shown in the description.
Owner:ZHEJIANG UNIV OF TECH
Synthesis method of 6-substituted phenanthridine compound
InactiveCN107793358AReduce consumptionRaw materials are easy to obtainOrganic chemistryOrganic solventSynthesis methods
The invention discloses a synthesis method of a 6-substituted phenanthridine compound represented by a formula (I), wherein a N-[1-phenyl-1'-biphenyl]benzenesulfonamide compound represented by a formula (II) is used as a raw materials and is subjected to a reaction in an organic solvent under the action of a [Fe] / Selectfluor catalyst to prepare a corresponding target product represented by the formula (I). According to the present invention, the synthesis method has advantages of environmental friendliness, mild reaction conditions, good functional group popularity, easy operation and the like, and uses the catalyst with advantages of low cost, easy availability and low toxicity. The formulas (I) and (II) are defined in the specification.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of 6-substituted phenanthridine compound
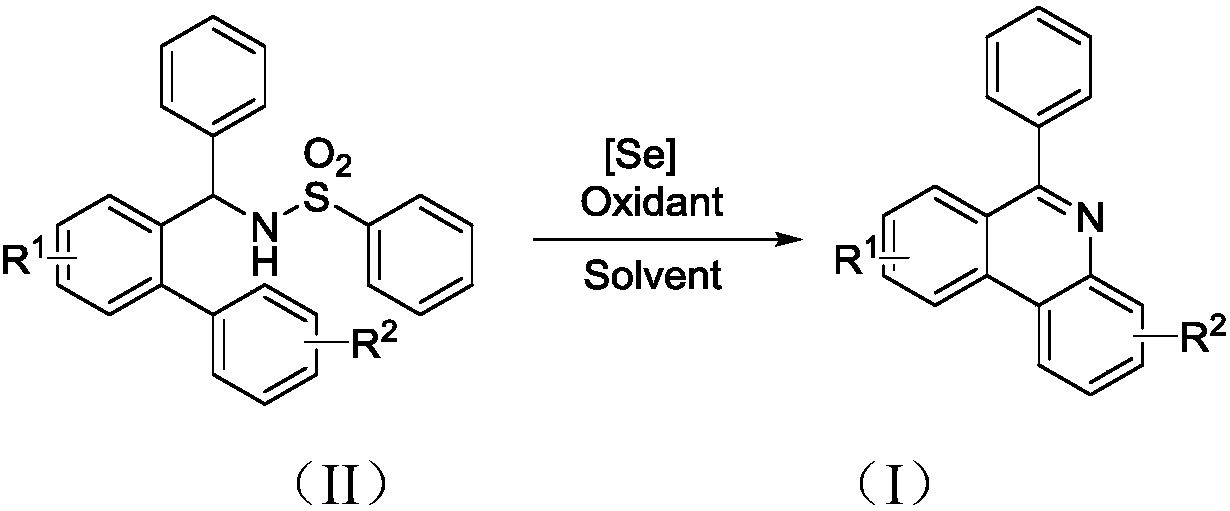
The invention discloses a preparation method of a 6-substituted phenanthridine compound represented by a formula (I), wherein a N-[1-phenyl-1'-biphenyl]benzenesulfonamide compound represented by a formula (II) is used as a raw materials and is subjected to a reaction in an organic solvent under the action of a [Se] / Selectfluor catalyst to prepare a corresponding target product represented by the formula (I). According to the present invention, the synthesis method has advantages of low harm on environment, relatively mild reaction conditions, energy consumption reducing, high product selectivity, easy operation and the like. The formulas (I) and (II) are defined in the specification.
Owner:ZHEJIANG UNIV OF TECH
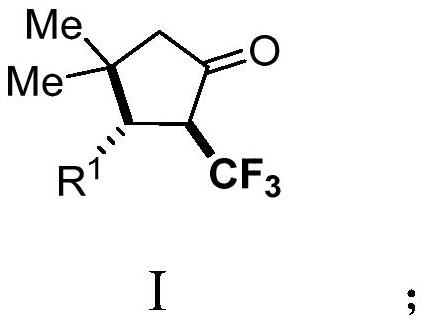
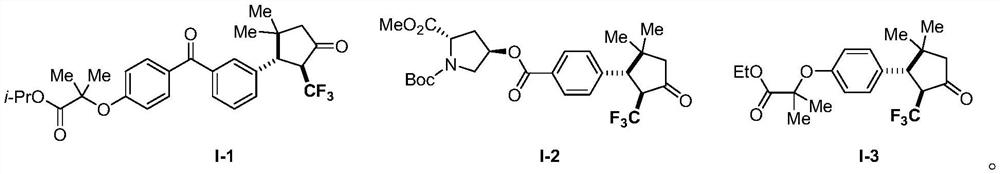
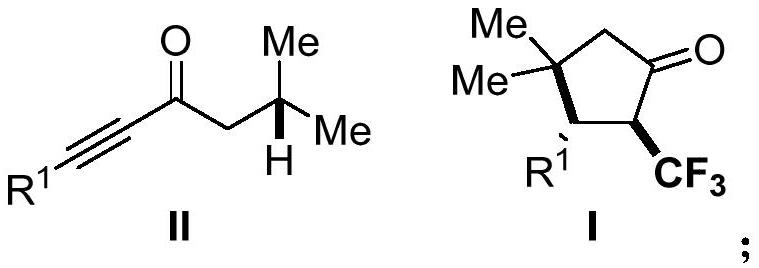
Derivative of 2-trifluoromethyl cyclopentanone and preparation method thereof
PendingCN114057578AImplement the buildAchieve trifluoromethylationOrganic compound preparationOrganic chemistry methodsAlkyneElectron transfer
The invention discloses a 2-trifluoromethyl cyclopentenone derivative and a preparation method of the 2-trifluoromethyl cyclopentenone derivative. The preparation method comprises the following steps: adding a photocatalyst and a trifluororeagent into N,N-dimethylformamide, then adding the reactant into an acetylenic ketone compound with a structure as shown in a formula II, and forming a reaction system in a certain reaction environment; and after the reaction is completed, performing post-treatment to obtain the 2-trifluoromethyl cyclopentanone derivative with the structure shown in the formula I in the claim 1. According to the ingenious design, carbonyl is used as a guiding group, a trifluoromethyl free radical region is selectively added to internal alkyne, and then hydrogen migration, 5-internal cyclization, electron transfer and proton transfer are carried out to start the reaction. Besides, the reaction condition is mild, the substrate application range is wide and the operation is simple. A new way is provided for synthesis of the complex 2-trifluoromethyl cyclopentanone derivative.
Owner:ZHEJIANG NORMAL UNIVERSITY
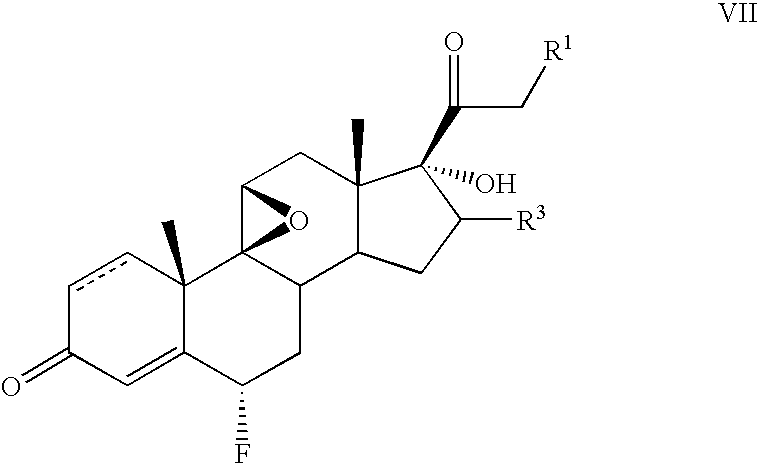
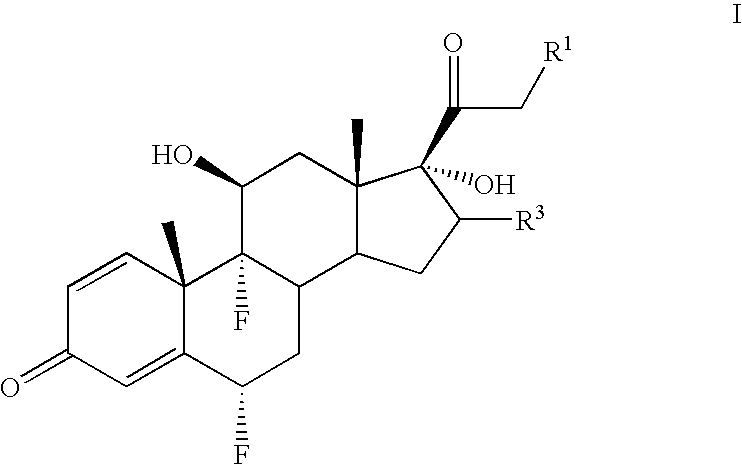
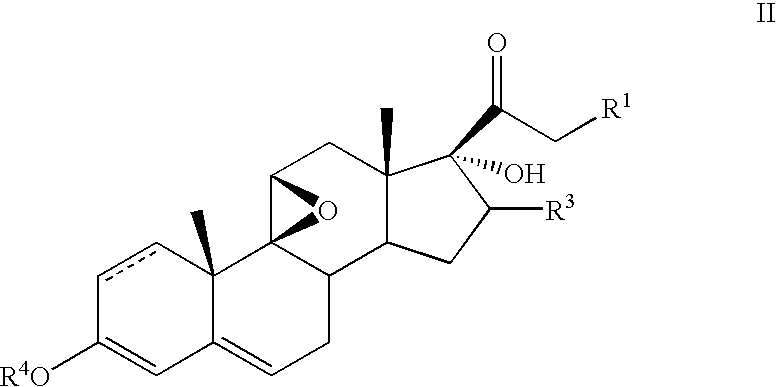
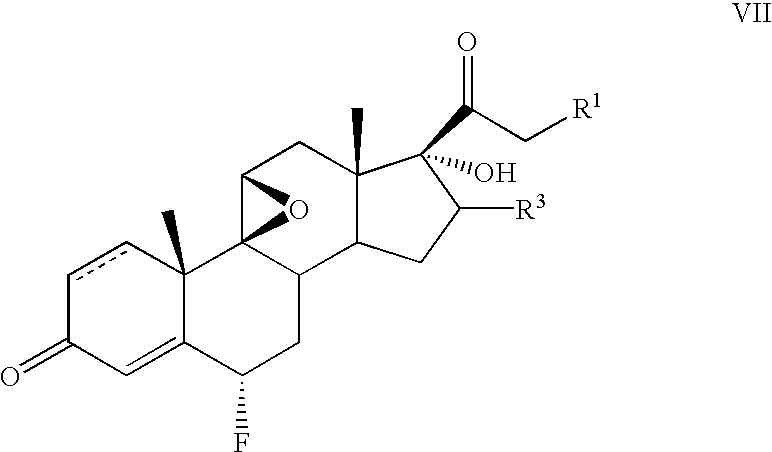
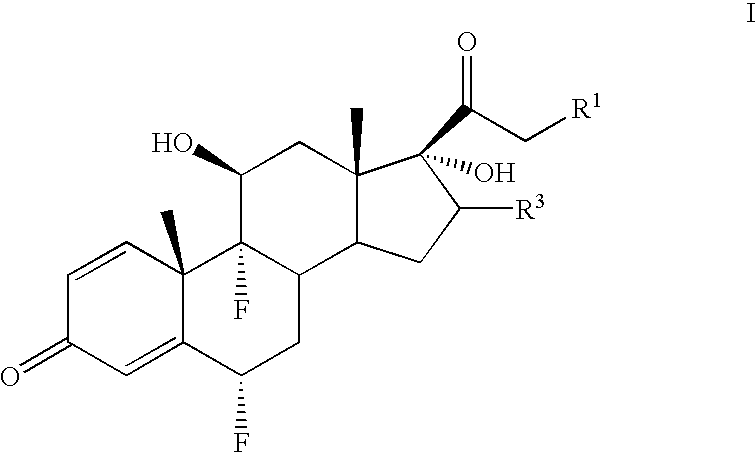
Method for the preparation of 6-α fluoro corticosteroids
InactiveUS7718793B2Safe and effective and stereoselective routeOrganic chemistry methodsSteroidsAcetic acidAryl
Owner:TARO PHARMA US INC
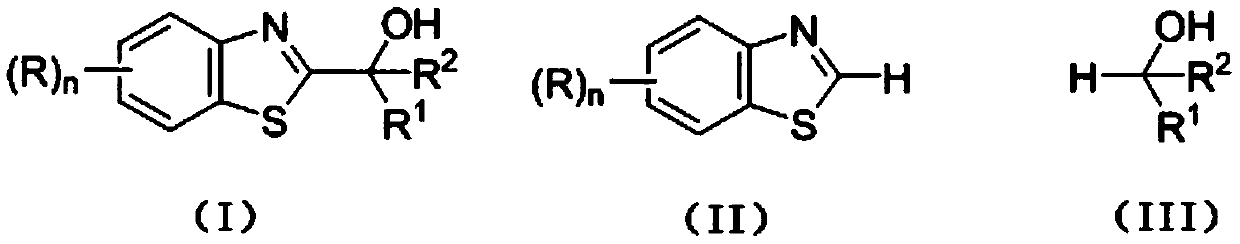
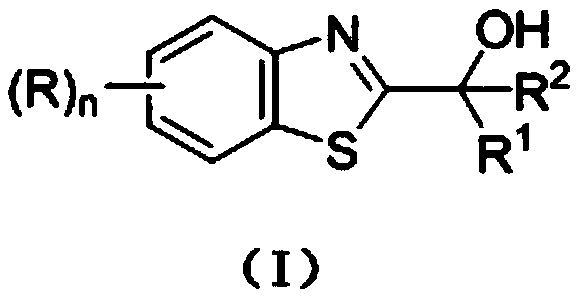
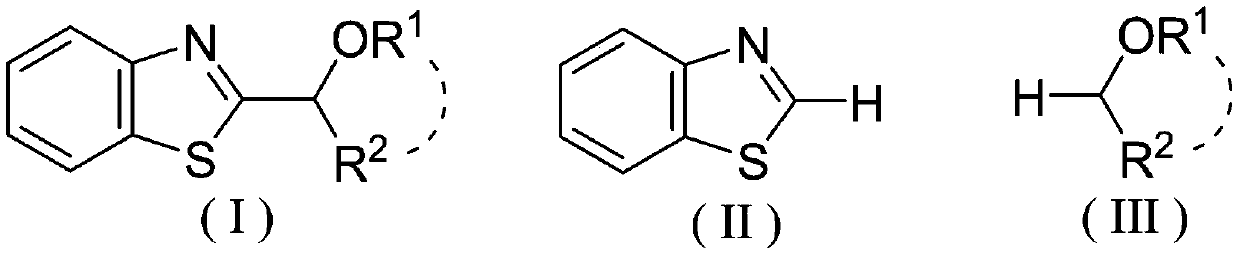
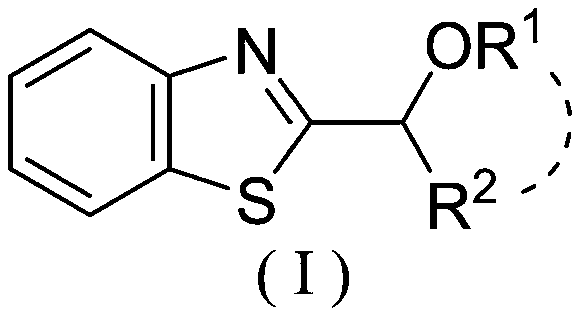
Photocatalytic synthesis method of C2 substituted 2H-benzothiazole hydroxyalkylated derivative
ActiveCN111170961AThe catalytic system is simpleMild reaction conditionsOrganic chemistrySelectfluorFatty alcohol
The invention discloses a photocatalytic synthesis method of a C2 substituted 2H-benzothiazole hydroxyl alkylation derivative. The method comprises the following steps of: mixing substituted 2H-benzothiazole shown as a formula (II) with fatty alcohol shown as a formula (III), adding an oxidizing agent Selectfluor, an additive trifluoroacetic acid and a solvent acetonitrile, and carrying out a normal temperature stirring reaction under the protection of nitrogen and the irradiation of an LED blue light lamp; carrying out TLC monitoring until the reaction is finished, and carrying out separationand purification on the reaction liquid to obtain the C2-substituted 2H-benzothiazole hydroxyalkylated derivative represented by formula (I). The invention provides a new method for synthesizing the2H-benzothiazole C2 hydroxyalkylated derivative through visible light induction by taking Selectfluor as an oxidizing agent, trifluoroacetic acid as an additive and acetonitrile as a solvent. The method is simple in catalytic system, mild in reaction condition and wide in substrate range.
Owner:ZHEJIANG UNIV OF TECH
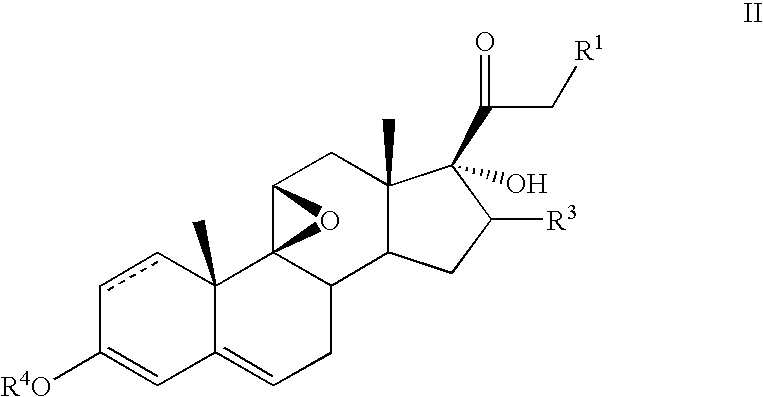
Method for the preparation of 6alpha-fluoro corticosteroids
InactiveUS7098328B2Safe and effective and stereoselective routeOrganic chemistry methodsSteroidsArylAcetic acid
A method for producing a 6α-fluorinated corticosteroid or derivative thereof by reacting a 17-hydroxy-21-ester epoxide of Formula IIwith a stereoselective fluorinating agent to stereoselectively form a 21-ester-17-hydroxy 6α-fluorinated compound of Formula VIIR1 can be OC(O)—Rd; R4 can be C(O)—Rd; R3 can be H or Rd. Each Rd may be the same or different and is independently selected from (C1-4)alkyl, aryl and heteroaryl. The dashed line can be a single or a double bond. R4 may be, for example, acetyl; R3 may be, for example, alpha or beta methyl; R1 may be, for example, acetate or propionate. The stereoselective fluorinating agent used in the reaction may be, for example, a fluoropyridinium or fluoroquinuclidium compound, for example, Selectfluor®.
Owner:TARO PHARMA US INC
Novel synthesis method of gem-difluoroalkane and alpha-fluorocarboxylic acid
InactiveCN109824472AEasy to operateRaw materials are simple and cheapCarboxylic acid nitrile preparationOrganic compound preparationSynthesis methodsCarboxylic acid
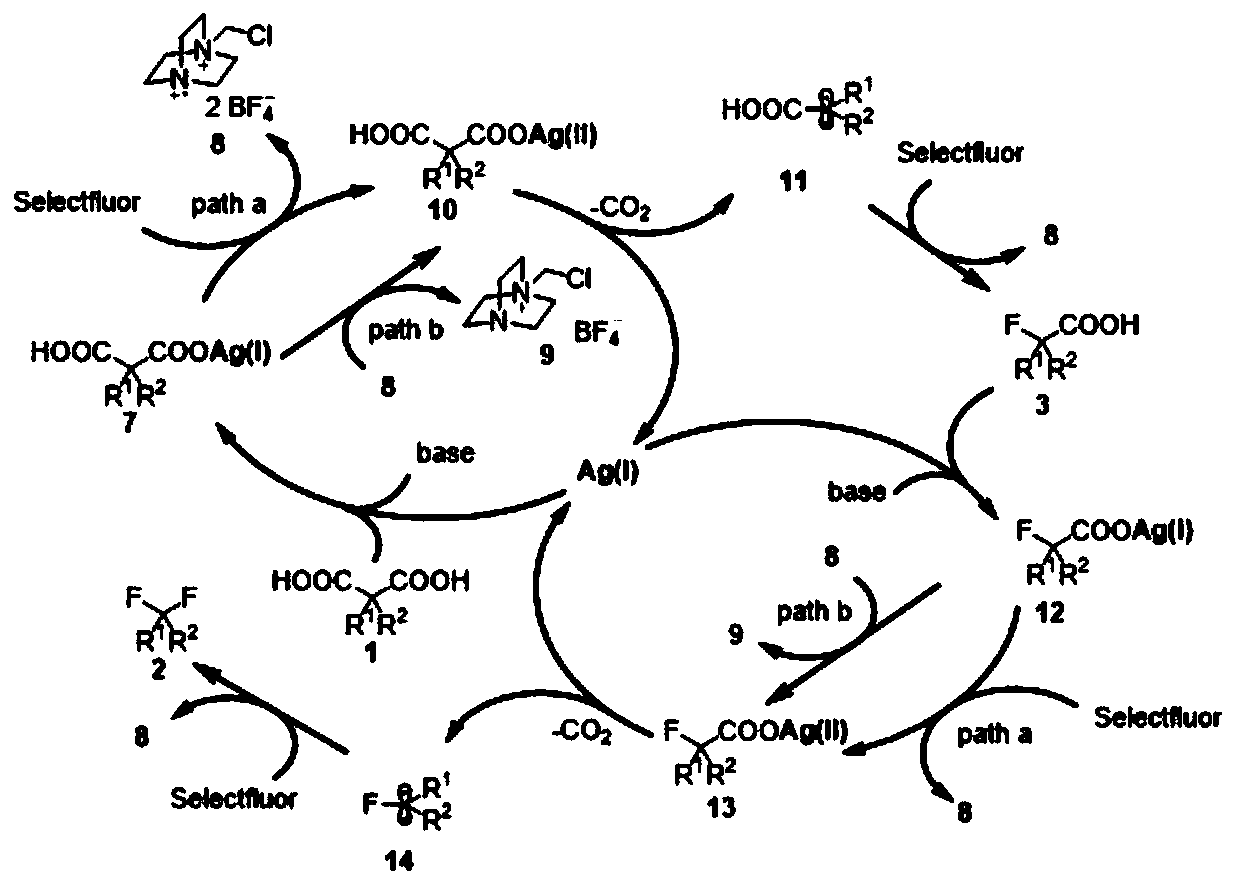
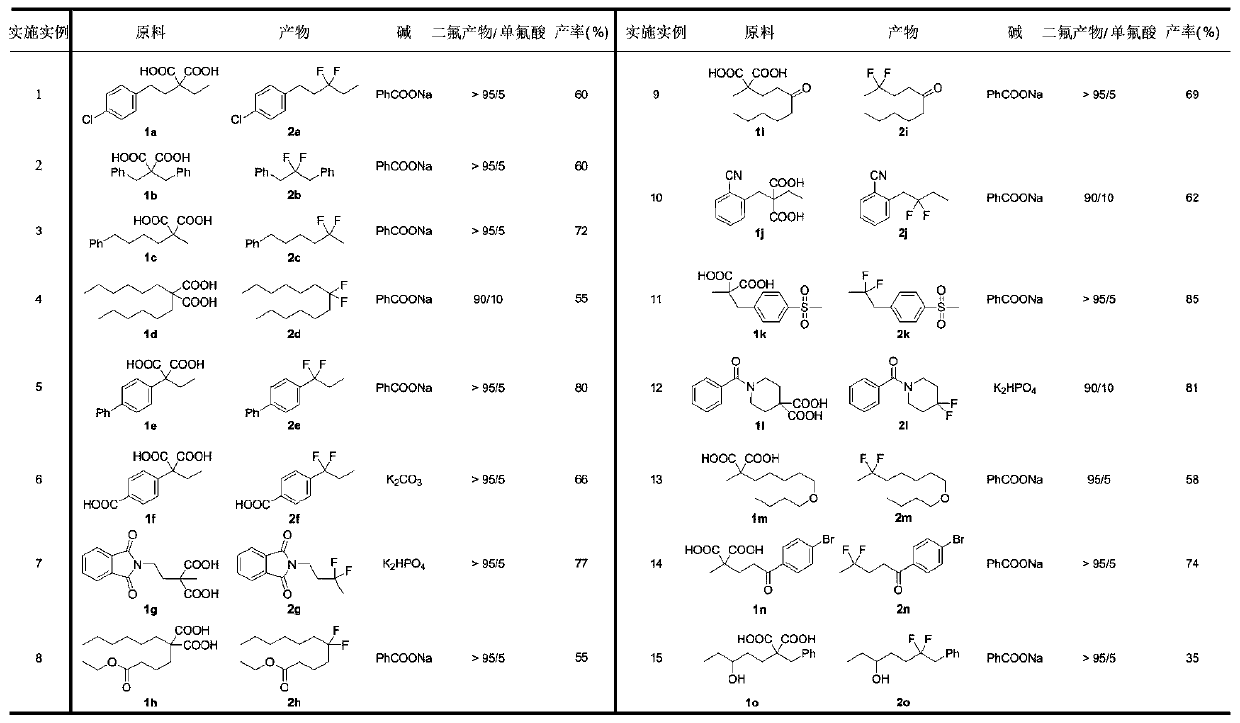
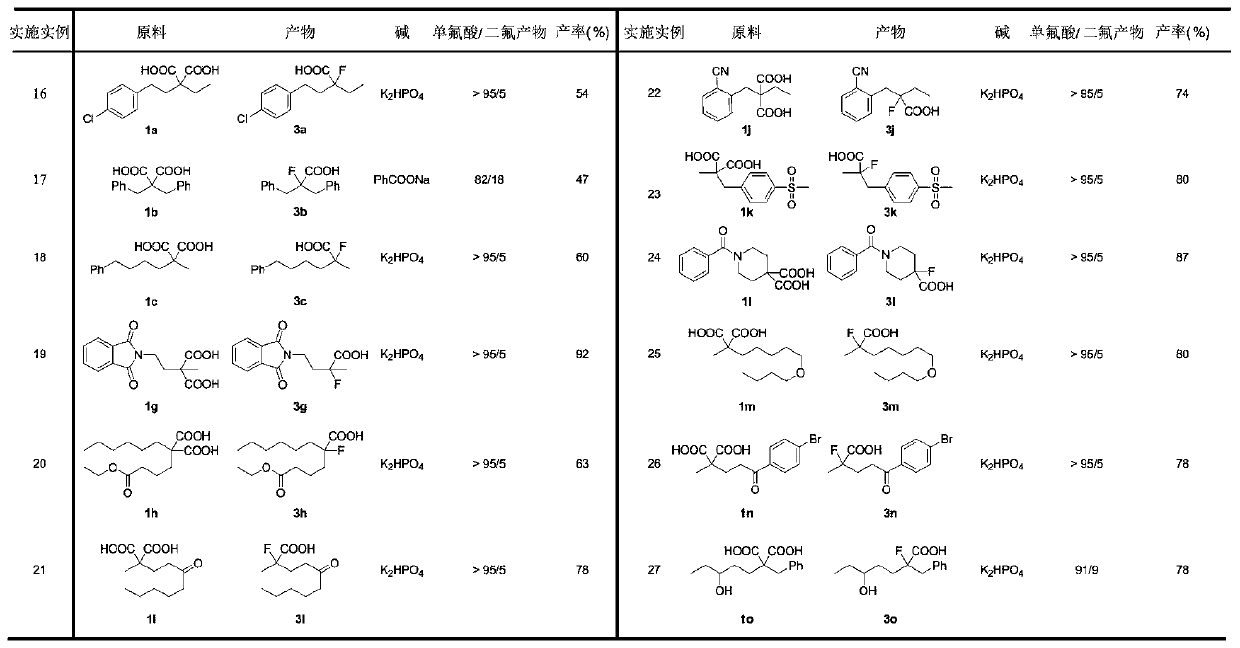
The invention discloses a novel synthesis method of gem-difluoroalkane and alpha-fluorocarboxylic acid. The synthesis method comprises following steps: step one, carrying out salt forming reactions between one carboxylic acid of propane diacid derivatives and monovalent silver to obtain silver carboxylate, and then oxidizing silver carboxylate by a fluorine reagent to obtain divalent silver salts;step two, oxidizing carboxylic acid by divalent silver salts to remove one CO2 molecule to form an alpha-carboxylic acid free radical; and step three, under a difluoro- condition, making alpha-fluorocarboxylic acid carry out decarboxylation and fluorination reactions to obtain gem-difluoroalkane. The invention relates to the technical field of organic fluorine compounds. According to the novel synthesis method, by adjusting the alkalis and solvents, under the catalytic action of monovalent silver, propane diacid derivatives R1C(COOH)2R2 and a fluorine reagent carry out reactions to selectively generate gem-difluoroalkane R1CF2R2 and alpha-fluorocarboxylic acid R1CF(COOH)R2; the operation is simple, the raw materials are cheap and can be easily prepared, the reaction conditions are mild, the chemical selectivity and the functional group compatibility are good, and the method is very practical.
Owner:NANJING UNIV OF TECH
New method for preparing N-n-butyl acrylamide
ActiveCN108675938AOrganic compound preparationCarboxylic acid amides preparationOrganic solventTetrafluoroborate
The invention relates to the technical field of fine chemical, and discloses a new method for preparing N-n-butyl acrylamide. The method comprises the specific steps: carrying out a stirring reactionof a starting raw material N-n-butyl-3-methylthiopropionamide and an additive Selectfluor 1-chloromethyl-4-fluoro-1,4-diazoniabicyclooctane bis(tetrafluoroborate) in an organic solvent 1,4-dioxane for8 h at the temperature of 100 DEG C, to obtain N-n-butyl acrylamide. The method has the following characteristics: N-n-butyl acrylamide can be obtained by directly heating and stirring with fluorineas an additive. Compared with the prior art, the method does not need to use poisonous, corrosion-prone and volatile acyl chloride as raw material and has simple operation and less environmental influence.
Owner:CHANGZHOU UNIV
Photocatalytic synthesis method of C2 substituted 2H-benzothiazole benzylated derivative
The invention discloses a photocatalytic synthesis method of a C2 substituted 2H-benzothiazole benzylated derivative. The photocatalytic synthesis method comprises the following steps of: mixing 2H-benzothiazole with substituted methyl benzene; adding an oxidizing agent Selectfluor, an additive trifluoroacetic acid and a solvent acetonitrile, carrying out a normal temperature stirring reaction under the protection of nitrogen and the irradiation of an LED blue light lamp, carrying out TLC monitoring until the reaction is finished, and carrying out separation and purification on the reaction liquid to obtain the C2 substituted 2H-benzothiazole benzylated derivative. The new method for synthesizing the C2 substituted 2H-benzothiazole benzylated derivative through visible light induction by taking Selectfluor as an oxidizing agent, trifluoroacetic acid as an additive and acetonitrile as a solvent is high in atom economy, simple in catalytic system, good in product yield, wide in substraterange and suitable for popularization and application.
Owner:ZHEJIANG UNIV OF TECH
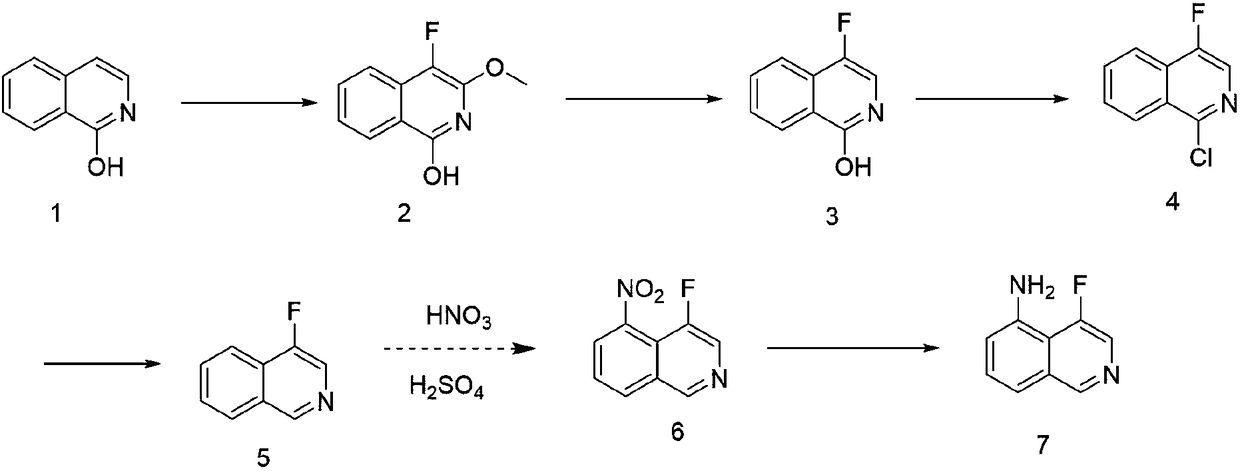
Synthetic method of 4-fluoroisoquinoline-5-amine
InactiveCN108558758ASolving expensive and hard-to-find problemsLow costOrganic chemistryIron powderIsoquinoline
The invention provides a synthetic method of 4-fluoroisoquinoline-5-amine. The synthetic method comprises the following steps: taking isoquinoline-1-ol as a raw material and reacting the isoquinoline-1-ol with Selectfluor to obtain 4-fluoro-3-methoxylisoquinoline-1-ol; dissolving the 4-fluoro-3-methoxylisoquinoline-1-ol in HCl and reacting to obtain 4-fluoroisoquinoline-1-ol and reacting the 4-fluoroisoquinoline-1-ol with phosphorus oxychloride to obtain 1-chloro-4-fluoroisoquinoline; obtaining 4-fluoroisoquinoline under the catalysis of a catalyst; reacting the 4-fluoroisoquinoline with concentrated nitric acid to obtain 4-fluoro-5-nitroisoquinoline; finally, under the acid condition, carrying out iron powder reduction to obtain the 4-fluoroisoquinoline-5-amine. The synthetic method of the 4-fluoroisoquinoline-5-amine, disclosed by the invention, has the advantages of concise route, reasonable technology, low cost of raw materials, simplicity and easiness in obtaining, convenience inoperation and post treatment, high total yield, no use of a poisonous reagent, easiness in enlargement and capability of producing the 4-fluoroisoquinoline-5-amine on a large scale.
Owner:SUZHOU KANGRUN PHARMA
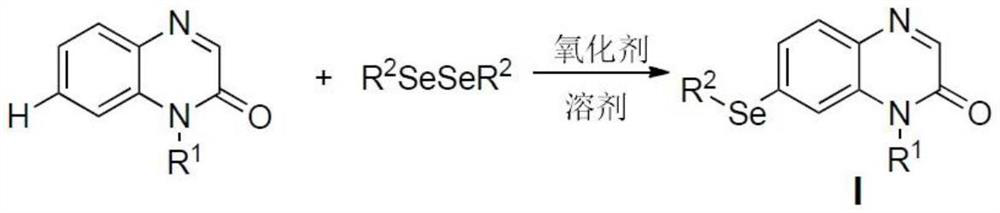
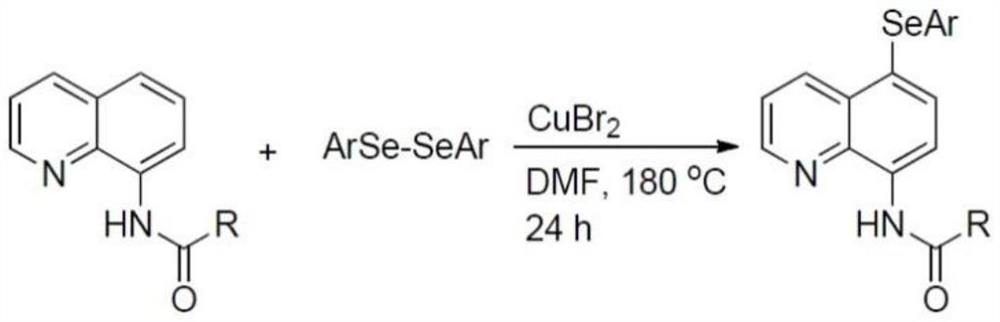
Method for regioselectively synthesizing 7-arylseleno quinoxalinone derivative
The invention discloses a preparation method of a 7-arylseleno quinoxalinone derivative (I), and belongs to the technical field of organic synthetic chemistry. The 7-arylseleno quinoxalinone derivative is synthesized by taking a substituted quinoxaline-2 (1H)-ketone derivative and diaryl diselenide as raw materials and a Selectfluor fluorine reagent and the like as an oxidizing agent without metalcatalysis. Compared with an existing synthesis method, the synthesis method has the following advantages that (1) the 7-arylseleno quinoxalinone derivative is synthesized in one step, raw materials and reagents are cheap and easy to obtain, the cost is low, and the application prospect is good; (2) only quinoxalinone 7-carbon with low electron cloud density is subjected to arylselenylation, and high regioselectivity is achieved; (3) the reaction conditions are mild, the reaction is carried out under the air condition, the yield is high, the operation is convenient, and the industrial production is facilitated. The derivative has potential application in the fields of medicine, chemical engineering, materials and the like, and a new way is provided for synthesis of the 7-arylseleno quinoxalinone derivative.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
Preparation method of 6-phenyl phenanthridine compound
The invention discloses a preparation method of 6-phenyl phenanthridine compound which is shown in a formula (I). According to the preparation method, N-(1-phenyl-1'-biphenyl)benzene sulfonamide shownin a formula (II) is utilized as a raw material, and the N-(1-phenyl-1'-biphenyl)benzene sulfonamide reacts in CH3CN under the action of a [Cu] / Selectfluor catalysome agent to prepare a correspondingtarget product formula (I). The synthesizing method disclosed by the invention has the advantages of low catalyst cost, small toxicity, environmental friendliness, moderate reaction condition, good functional group popularization, convenience in operation and the like. The formula (I) and the formula (II) are shown in the description.
Owner:ZHEJIANG UNIV OF TECH
C2 substituted 2H-benzothiazole aryl acylated derivative, and synthesis method and application thereof
ActiveCN113072520AInhibits topoisomeraseGood chemical stabilityOrganic chemistryAntineoplastic agentsAir atmospherePtru catalyst
The invention discloses a C2 substituted 2H-benzothiazole aryl acylated derivative, and a synthesis method and application thereof. The preparation method of the derivative comprises the following steps: mixing substituted 2H-benzothiazole and substituted methyl benzene, adding into a solvent, in the presence of an oxidizing agent Selectfluor and an additive trifluoroacetic acid, carrying out a heating and stirring reaction in the air atmosphere, performing TLC monitoring is conducted till the reaction is finished, and separating and purifying the obtained reaction liquid to obtain the target product C2 substituted 2H-benzothiazole aryl acylated derivative. The substituted methyl benzene used in the invention is low in price, easy to obtain and good in chemical stability, the method has the advantages of being high in atom economy, simple in catalytic system, free of transition metal catalysts, good in product yield, wide in substrate range and the like, and the prepared compound structure can be further optimized as an anti-tumor drug lead.
Owner:ZHEJIANG UNIV OF TECH
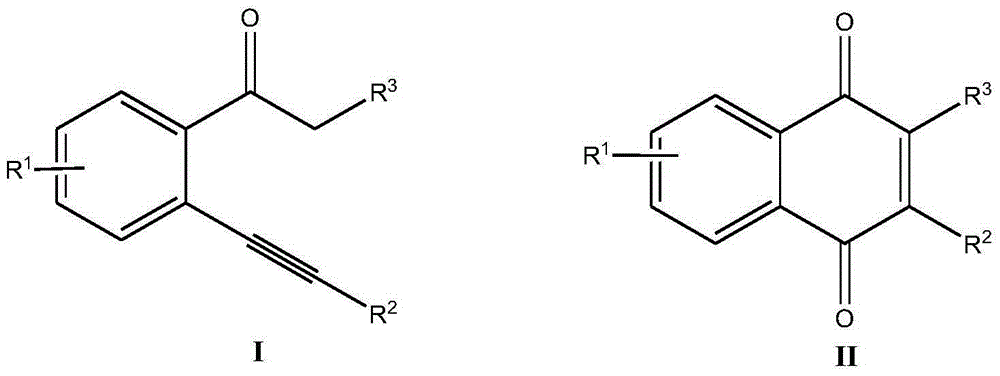
Method for synthesizing 2-substituted-1,4-naphthoquinone compound
ActiveCN106316819AReduce consumptionRaw material variableQuinone preparation by oxidationOxygenAcetophenone
The invention discloses a method for synthesizing a 2-substituted-1,4-naphthoquinone compound. The method comprises the following steps: by taking a 2-alkynyl acetophenone compound of a formula I as shown in the specification as a raw material, in the presence of a copper catalyst, by taking Selectfluor as an oxidant, adding an alkali substance, performing a stirring reaction for 10-24 hours at 70 DEG C in a mixed solvent of acetonitrile and water, and after the reaction is completed, and performing aftertreatment on reaction liquid, thereby obtaining the 2-substituted-1,4-naphthoquinone compound of a formula II as shown in the specification. By adopting the method disclosed by the invention, the raw materials are variable, a great amount of derivatives can be generated, the catalyst is cheap and easy to obtain, the cost can be greatly lowered, and no pollution can be caused; oxygen sources used in the method are water and Selectfluor; the reaction condition is gentle, and energy consumption can be reduced; in addition, the method has the characteristics of being high in yield, good in substrate universality, simple and convenient in aftertreatment operation, and the like.
Owner:ZHEJIANG UNIV OF TECH
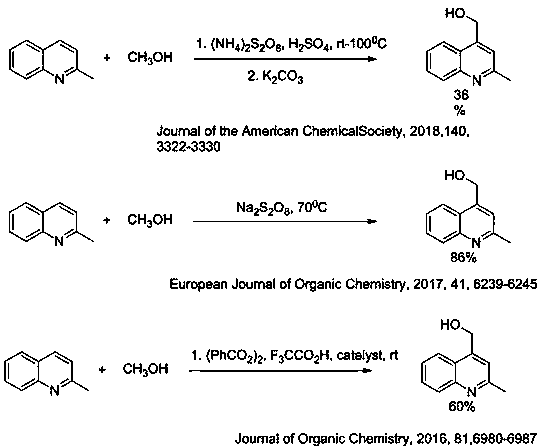
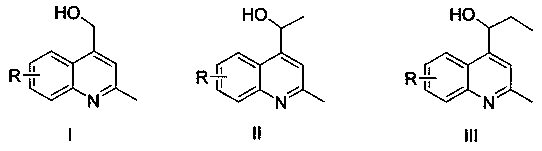
Preparation method of 2-methyl-4-(tetrahydrofuran-2-base) quinoline derivative
The invention belongs to the technical field of chemical synthesis, and particularly relates to a 2-methyl-4-(tetrahydrofuran-2-base) quinoline derivative. The method comprises the following steps: reacting 2-methyl quinoline and its derivatives with tetrahydrofuran in water phase catalyzed by Selectfluor / AgNO3 to obtain a 2-methyl-4-(tetrahydrofuran-2-base) quinoline derivative, a product of dehydrogenation coupling of the 4-position of the 2-methyl quinoline derivative and the 2-psition of the tetrahydrofuran of the 2-methyl quinoline derivative through column chromatography. Carried out ina mixture of water and tetrahydrofuran under the catalysis of Selectfluor / AgNO3, the preparation method of the 2-methyl-4-(tetrahydrofuran-2-base) quinoline derivative has the advantages of good solubility, wide applicability, high yield of reaction and strong controllability. The preparation method of the 2-methyl-4-(tetrahydrofuran-2-base) quinoline derivative is green and environmental protection, less by-reaction products, green and high efficiency.
Owner:UNIV OF JINAN
Preparation method of methyl 4-(dimethoxymethyl)quinoline-2-carboxylate derivative
The invention belongs to the technical field of chemical synthesis, and particularly relates to a preparation method of a methyl 4-(dimethoxymethyl)quinoline-2-carboxylate derivative. The method is realized by the following steps that under catalysis of Selectfluor / AgNO3, methyl quinoline-2-carboxylateor a derivative thereof reacts with methanol, and a methyl 4-(dimethoxymethyl)quinoline-2-carboxylate derivative is obtained after column chromatography. The method provided by the invention is carried out in methanol solution under the catalysis of Selectfluor / AgNO3, and has the advantages of good substrate solubility, wide applicability, high reaction yield and high controllability. The preparation method provided by the invention is environment-friendly, has few side reaction products, andhas high efficiency.
Owner:UNIV OF JINAN
Preparation method of fluoride chalcone
InactiveCN106699530AMild reaction conditionsLow priceOrganic compound preparationCarbonyl compound preparationMetal catalystWhite powder
The invention discloses a preparation method of fluoride chalcone. The preparation method comprises the following steps: firstly, mixing acetonitrile with methanol to obtain a reaction solvent; secondly, adding chalcone and an electrophilic fluorinating agent Selectfluor, and carrying out heating and stirring reaction; after the reaction is ended, generating yellow liquid, and filtering out white powder; heating to evaporate the reaction solvent, separating a reaction solution by a column chromatographic method, and drying to obtain a solid product fluoride chalcone of which the highest yield can reach 60 percent. The preparation method disclosed by the invention has the advantages of simplicity, directness, mild reaction conditions and no need of harsh reaction environment or metal catalyst; a main reaction can be finished by a one-step one pot method; a fluoride source is safe and environment-friendly and low in price, so that the synthesis cost is greatly reduced; the preparation method is suitable for large-scale industrial production.
Owner:NANJING UNIV OF SCI & TECH
Mild photocatalytic synthesis method of C2 ether substituted 2H-benzothiazole derivative
InactiveCN111253334AThe catalytic system is simpleMild reaction conditionsOrganic chemistryPhoto catalysisSelectfluor
The invention discloses a mild photocatalytic synthesis method of a C2 ether substituted 2H-benzothiazole derivative. The preparation method comprises the following steps: mixing 2H-benzothiazole shown as a formula (II) with ether shown as a formula (III); adding an oxidizing agent Selectfluor, an additive trifluoroacetic acid and a solvent acetonitrile, carrying out a normal temperature stirringreaction under the protection of nitrogen and the irradiation of an LED blue light lamp, carrying out TLC monitoring until the reaction is finished, and carrying out separation and purification on thereaction liquid to obtain the C2 ether substituted 2H-benzothiazole derivative represented by the formula (I). The invention provides a new method for synthesizing the C2 ether substituted 2H-benzothiazole derivative through visible light induction by taking Selectfluor as an oxidizing agent, trifluoroacetic acid as an additive and acetonitrile as a solvent, and the method has the advantages of simple catalytic system, mild reaction conditions, wide substrate range and the like.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of 4-(1,4-dioxan-2-yl) quinoline-2-methyl formate derivatives
The invention belongs to the technical field of chemical synthesis, in particular to a preparation method of 4-(1,4-dioxan-2-yl) quinoline-2-methyl formate derivatives. The method is realized by the following steps: under the catalysis of Selectfluor / FeSO4.7H2O, allowing quinoline-2-methyl formate and its derivatives to react with 1, 4-dioxane, performing column chromatography to obtain 4-(1,4-dioxan-2-yl) quinoline-2-methyl formate derivatives. The method provided by the invention is that Selectfluor / FeSO4.7H2O is used for catalyzing, 1,4-dioxan reacts, the substrate solubility is good and the applicability is wide; and the yield of the reaction is high and the controllability is strong. The method provided by the invention has the advantages of environmental protection, less by-reactionproducts, green and high efficiency.
Owner:UNIV OF JINAN
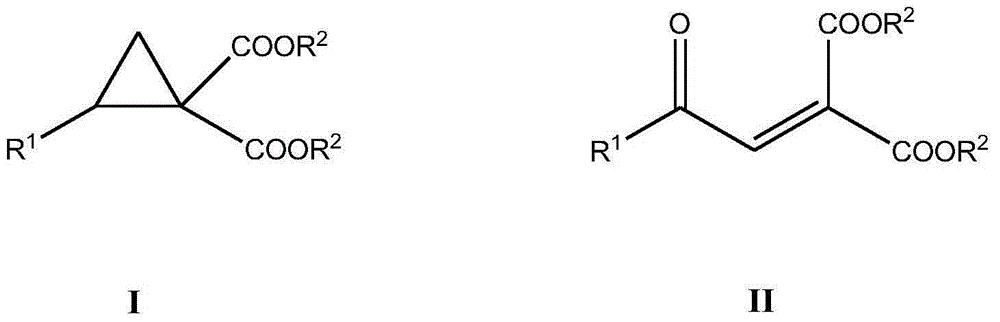
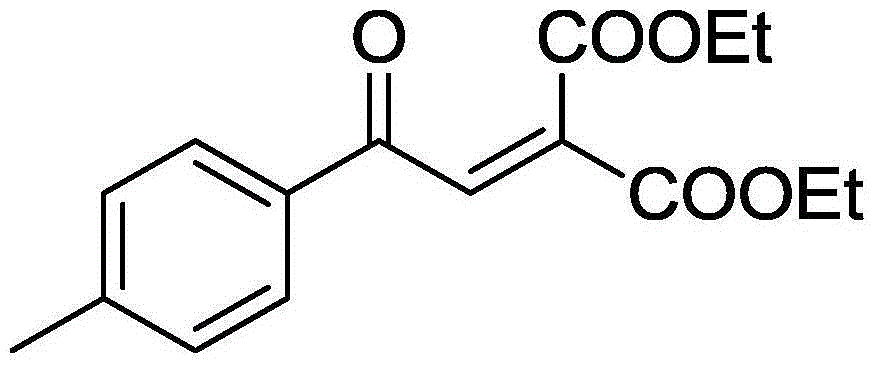
Method for synthesizing benzoyl methylene malonic ester compound
ActiveCN106349073AImprove universalityLow costOrganic compound preparationCarboxylic acid esters preparationAcetonitrileSolvent
The invention discloses a method for synthesizing a benzoyl methylene malonic ester compound of a formula II as shown in the description. The method comprises the following steps: by taking a diester cyclopropane compound of a formula I as shown in the description as a raw material, under the action of an iron catalyst, by taking Selectfluor as an oxidant, in the presence of an acetonitrile solvent, performing stirring reaction for 8-16 hours at 70-90 DEG C, and after the reaction is completed, performing aftertreatment on a reaction liquid to obtain the benzoyl methylene malonic ester compound of the formula II as shown in the description. The method disclosed by the invention has the advantages that the raw materials are low in price and easily accessible, the process is simple, the operation is simple and convenient, environmental friendliness is achieved, the yield is high, and the functional group universality is good.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of 2-fluoromethyl quinoline derivative
The invention belongs to the technical field of chemical synthesis, and particularly relates to a preparation method of a 2-fluoromethyl quinoline derivative. The method is implemented through the following steps: reacting 2-methylquinoline and a derivative thereof with Selectfluor in a DMF solution, and performing column chromatography to obtain a monofluoro-2-methylquinoline derivative. The method provided by the invention is carried out in the DMF solution and has good substrate solubility and wide applicability; and the reaction yield is high and the controllability is strong. The method provided by the invention is green and environment-friendly, has few side reaction products, and is green and efficient.
Owner:UNIV OF JINAN
Preparation method of 2-methyl-4-hydroxymethyl quinoline and derivatives thereof
The invention belongs to the technical field of chemical synthesis, and particularly relates to a method for reacting 2-methylquinoline and derivatives thereof with primary alcohol. The method is carried out by the following steps of: under the catalysis of Selectfluor / AgNO3, reacting the 2-methylquinoline and the derivatives in a primary alcohol aqueous solution, and performing column chromatography to obtain a product of dehydrogenation coupling of 4-position and alcohol compounds of the 2-methylquinoline derivatives. The method provided by the invention is carried out in a mixed solution ofwater and alcohol compounds under the catalysis of Selectfluor / AgNO3, and has the advantages of good substrate solubility, wide applicability, high reaction yield and strong controllability. The method is green and environment-friendly, and has the advantages of few side reaction products, green and high efficiency.
Owner:UNIV OF JINAN
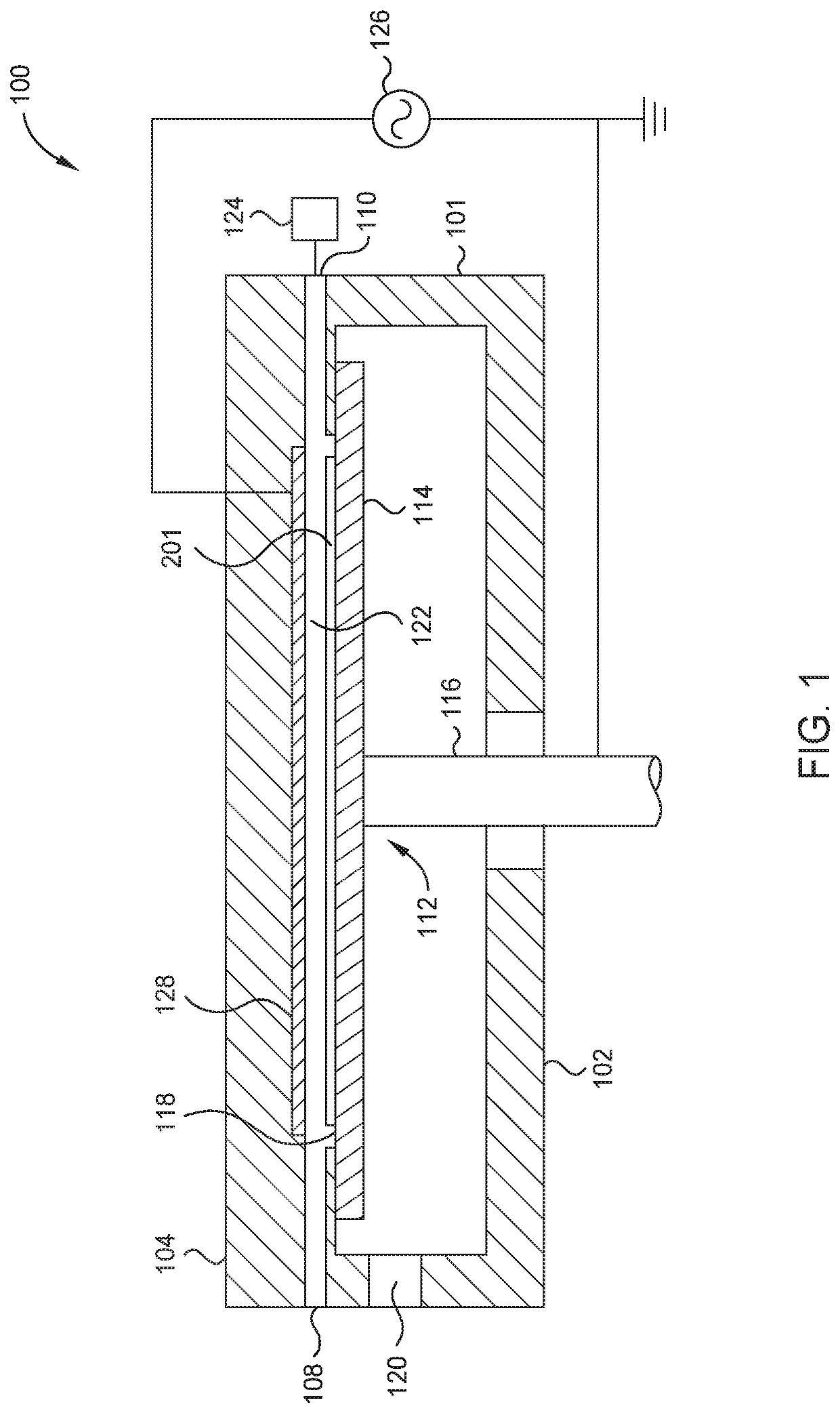
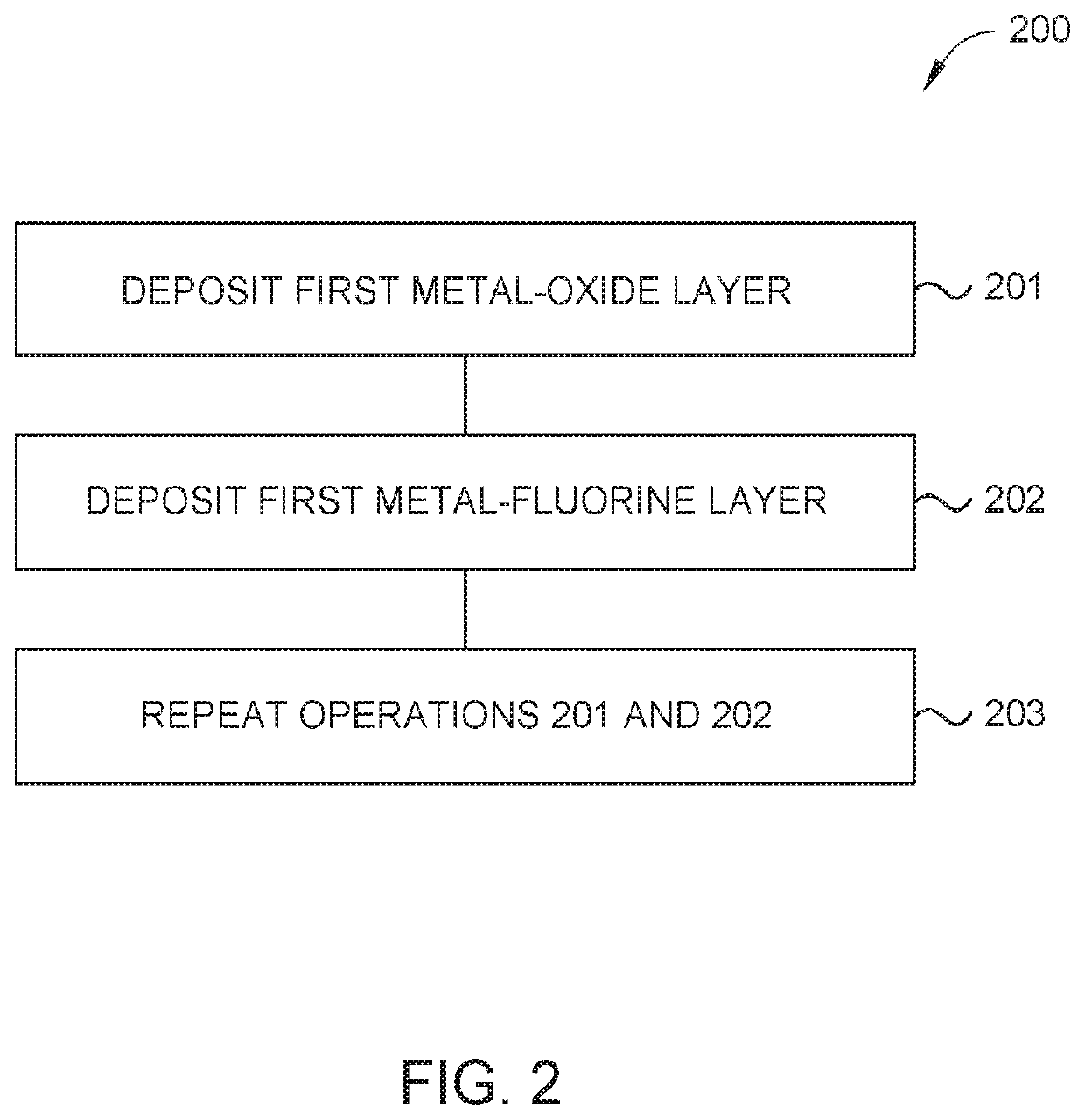
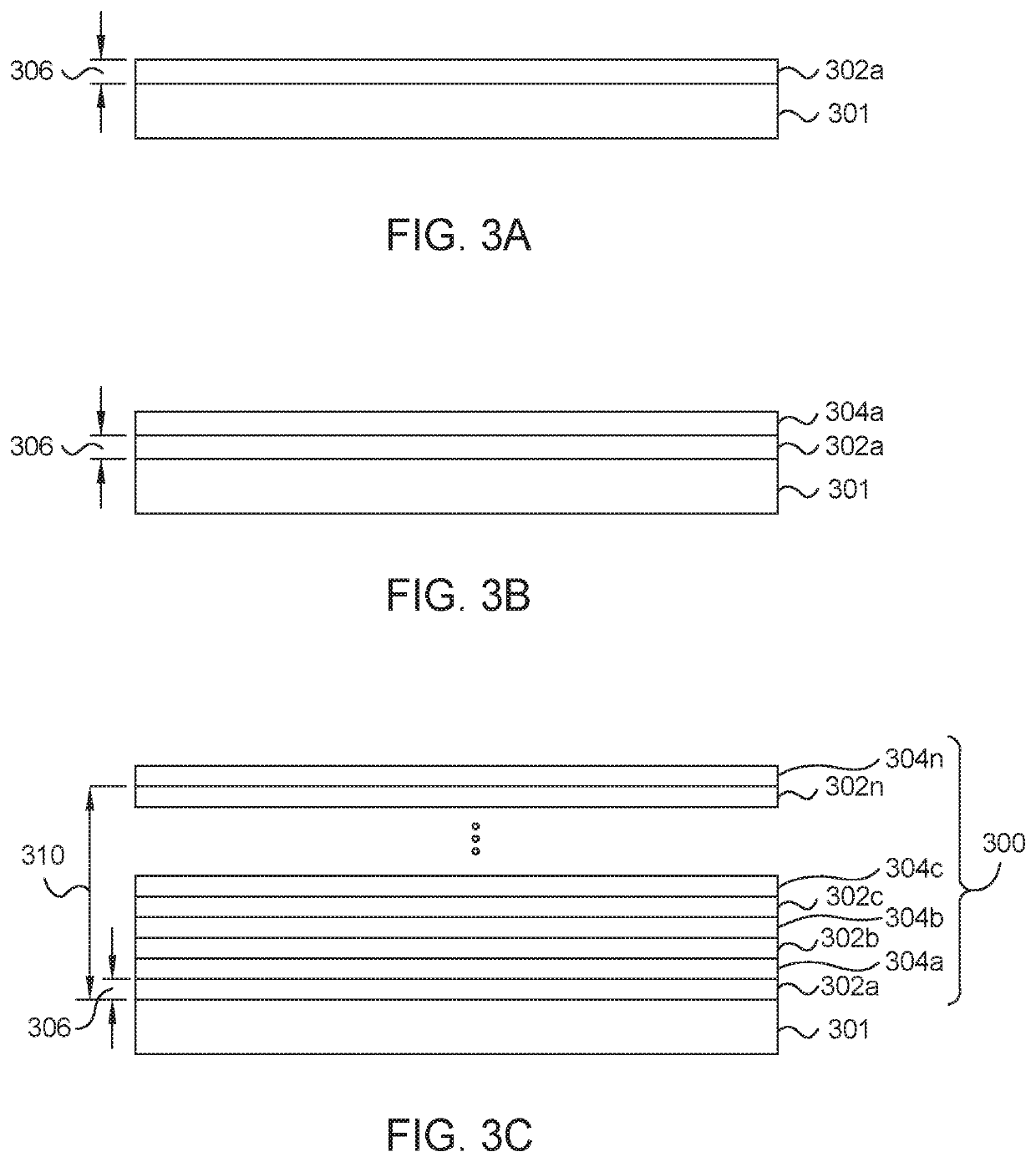
Oxy-fluoride compounds for chamber parts protection
ActiveUS20200283897A1Electric discharge tubesChemical vapor deposition coatingFluorideMetal membrane
Embodiments described herein provide a method of forming amorphous a fluorinated metal film. The method includes positioning an object in an atomic layer deposition (ALD) chamber having a processing region, depositing a metal-oxide containing layer on an object using an atomic layer deposition (ALD) process, depositing a metal-fluorine layer on the metal-oxide containing layer using an activated fluorination process, and repeating the depositing the metal-oxide containing layer and the depositing the metal-oxide containing layer until a fluorinated metal film with a predetermined film thickness is formed. The activated fluorination process includes introducing a first flow of a fluorine precursor (FP) to the processing region. The FP includes at least one organofluorine reagent or at least one fluorinated gas.
Owner:APPLIED MATERIALS INC
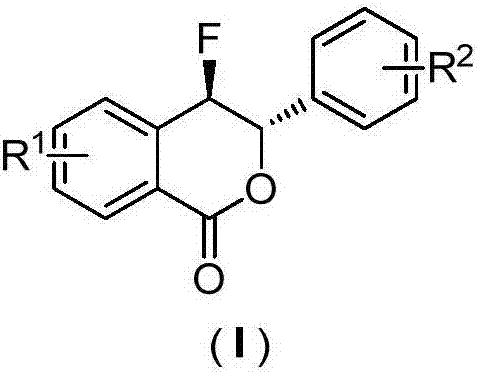
Preparation method of fluoro-3,4-dihydroisocoumarin derivative
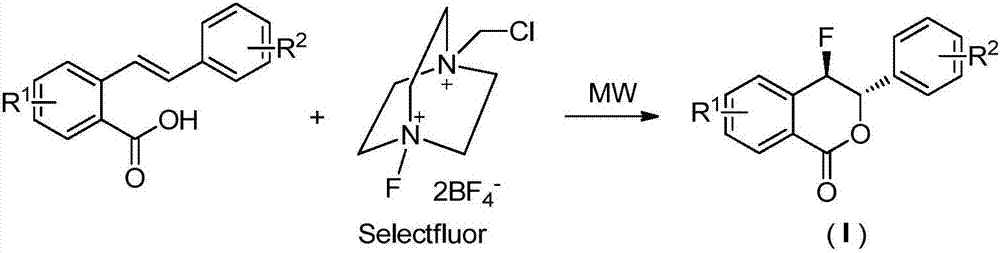
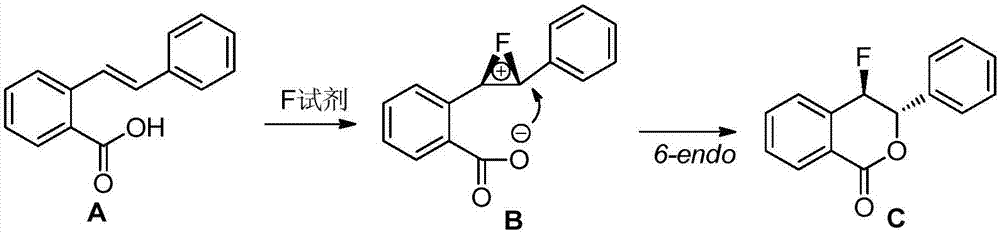
ActiveCN106866608ARaw materials are cheap and easy to getMild reaction conditionsOrganic chemistryBenzoic acidMicrowave
The invention discloses a preparation method of a fluoro-3,4-dihydroisocoumarin derivative (I), and belongs to the field of organic chemistry. According to the method, under the assistance of microwaves, substituted o-vinylphenol benzoic acid and Selectfluor fluoride agents are used as raw materials to take a reaction in a solvent to synthesize a fluoro-3,4-dihydroisocoumarin derivative. The formula (I) is shown as the accompanying drawing. Compared with the existing synthesis method, the method provided by the invention has the following advantages that (1) raw materials are cheap and can be easily obtained; the fluoro-3,4-dihydroisocoumarin derivative is synthesized in one step; the cost is low; good application prospects are realized; (2) the reaction conditions are mild; the reaction is performed under the air condition; the yield is high; the operation is convenient; green and environment-friendly effects are achieved; the industrial production is facilitated. A novel path is provided for the synthesis of the fluoro-3,4-dihydroisocoumarin derivative.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
Synthesizing method of methyl phenyl sulfoxide
ActiveCN107814756ASimple process conditionsQuick responseOrganic chemistryOrganic compound preparationMetal catalystNitrogen
The invention discloses a synthesizing method of methyl phenyl sulfoxide. The method is characterized in that a thioanisole derivate is used as a raw material, under nitrogen condition, acetonitrile is used as a solvent, and SelectFluor TM is used as an oxidizing reagent, the reaction is performed for 10-20min at room temperature, then triethylamine is added for continuously catalyzing for 10-20min; the reacting liquid is separated and purified to obtain a dimethyl sulfoxide compound after the reaction is finished. According to the synthesizing method, technical conditions are mild, the operation is simple, the reaction time is extremely short, conversion rate and yield are stable, and the substrate range is wide; the SelectFluor TM is used as the oxidizing reagent, so that a metal catalyst is saved, and the method is an efficient method for synthesizing the dimethyl sulfoxide compound.
Owner:NANJING UNIV OF SCI & TECH
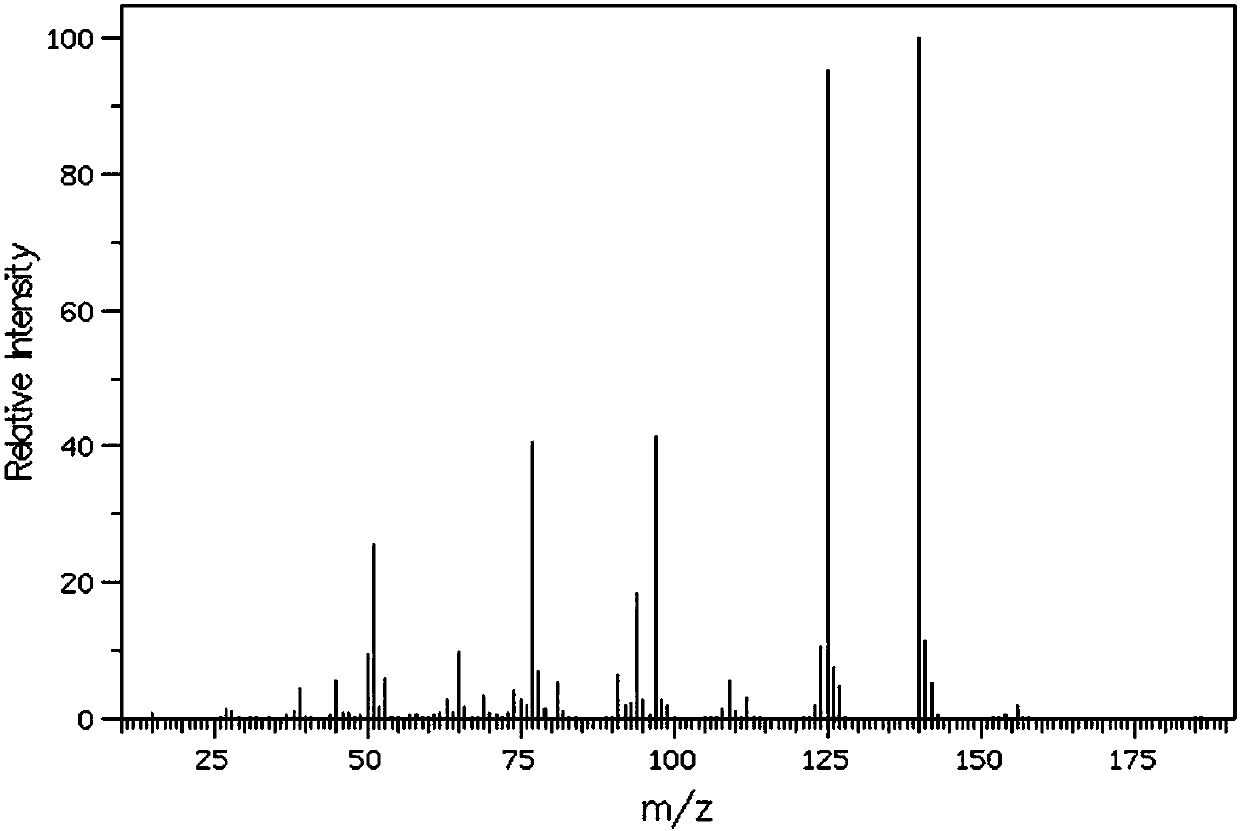
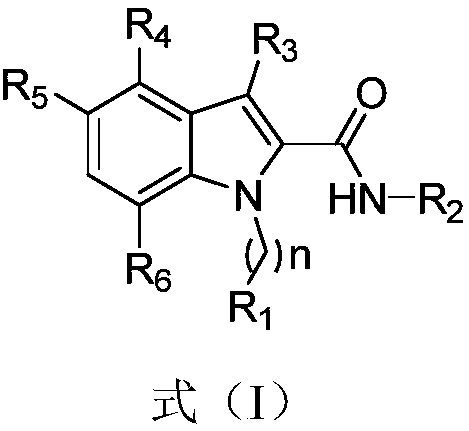
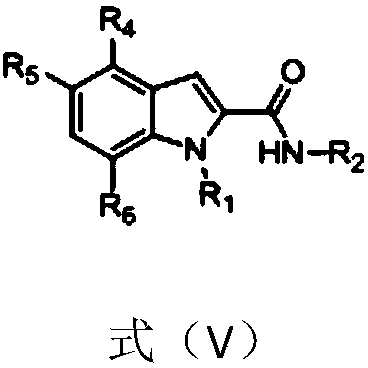
1H-indole-2-carboxamide derivative and preparation method and applications thereof
InactiveCN108794379AHigh affinityHigh activityNervous disorderOrganic chemistryImmunologic disordersDisease
The invention belongs to the technical field of medicine, and discloses a 1H-indole-2-carboxamide derivative of a formula (I) and a preparation method thereof. The preparation method includes the following steps: a compound of a formula (II) and sodium hydroxide are subjected to a hydrolysis reaction to synthetize a compound of a formula (III); the compound of the formula (III) and H2NR2 are subjected to an amidation reaction to synthetize a compound of a formula (IV); the compound of the formula (IV) and halogenated R1 are subjected to a nucleophilic substitution reaction to synthetize a compound of a formula (V); the compound of the formula (V) and a selectfluor are subjected to an electrophilic substitution reaction to synthetize a compound of a compound (VI), and the compound of the formula (VI) and the halogenated R1 are subjected to the nucleophilic substitution reaction to synthetize the compound of the formula (I). The 1H-indole-2-carboxamide derivative of the formula (I) is aagonist with high affinity, selectivity and activity for CB2 receptors, and can be potentially used for treating a plurality of diseases such as multiple sclerosis, autoimmune diseases, osteoporosis,arthralgia, inflammatory pain, and neurodegenerative diseases.
Owner:EAST CHINA NORMAL UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com