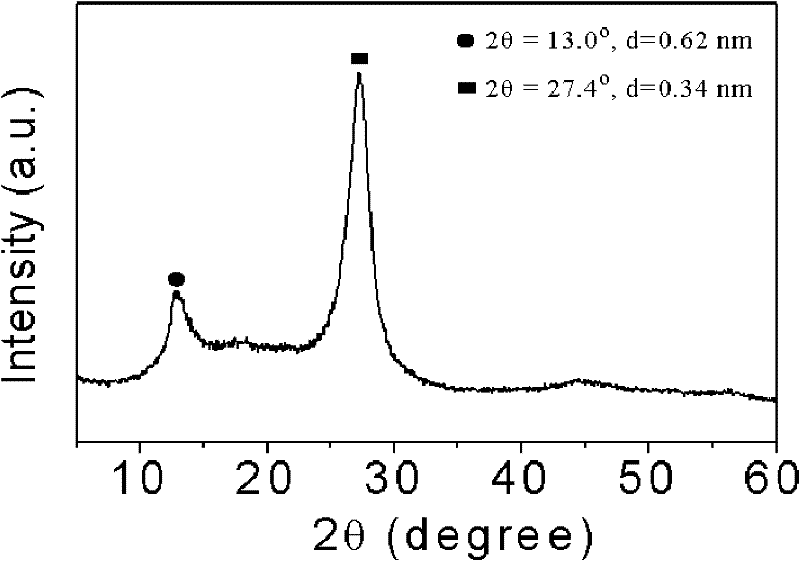
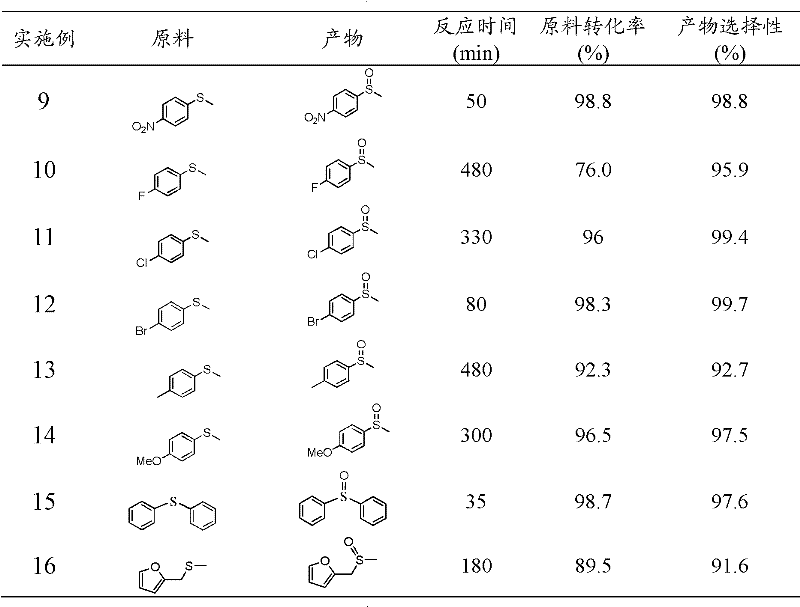
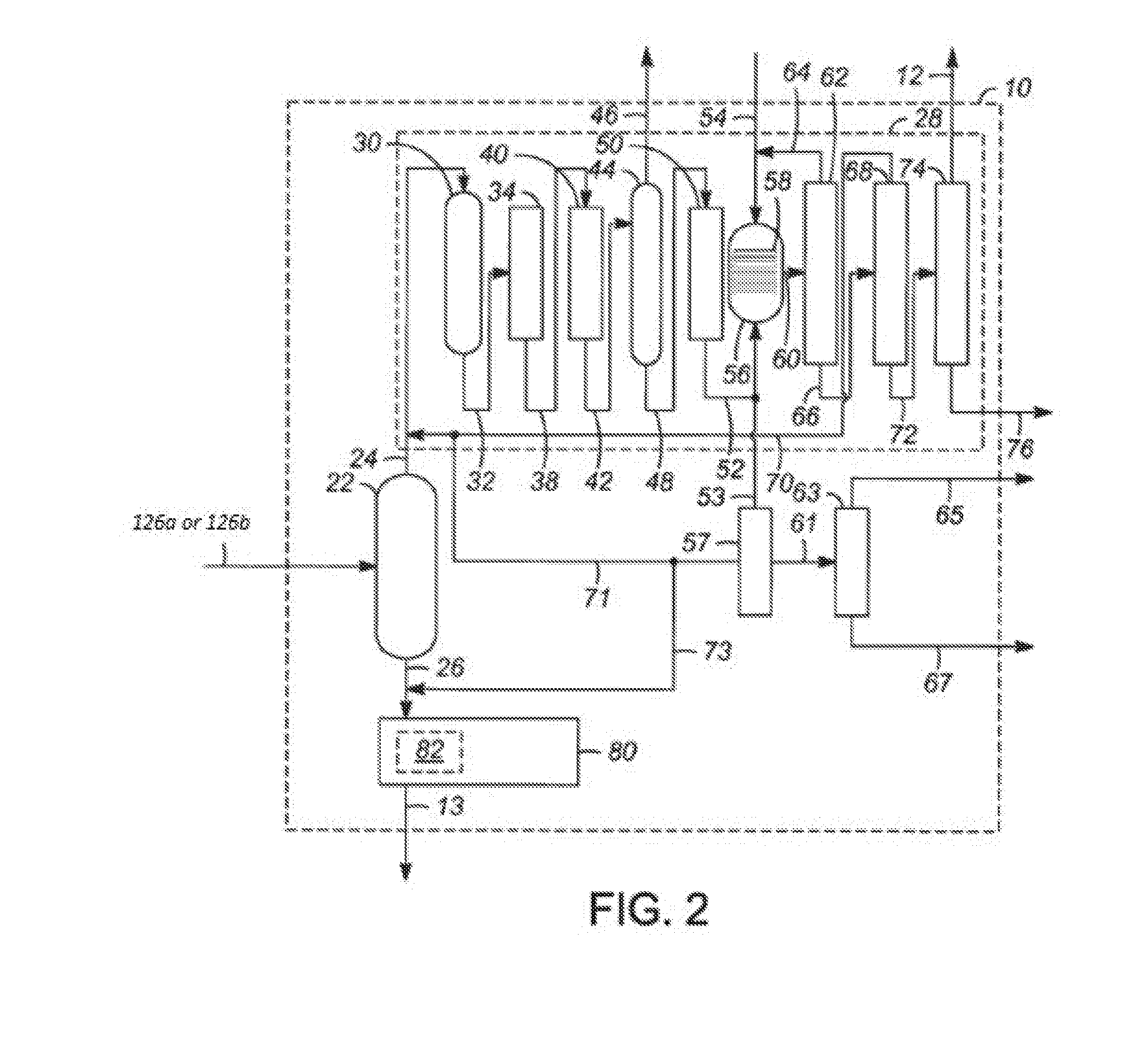
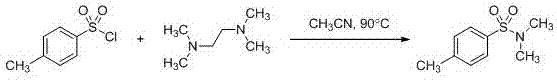
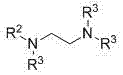
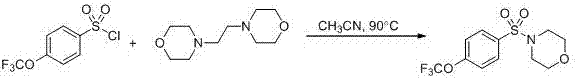
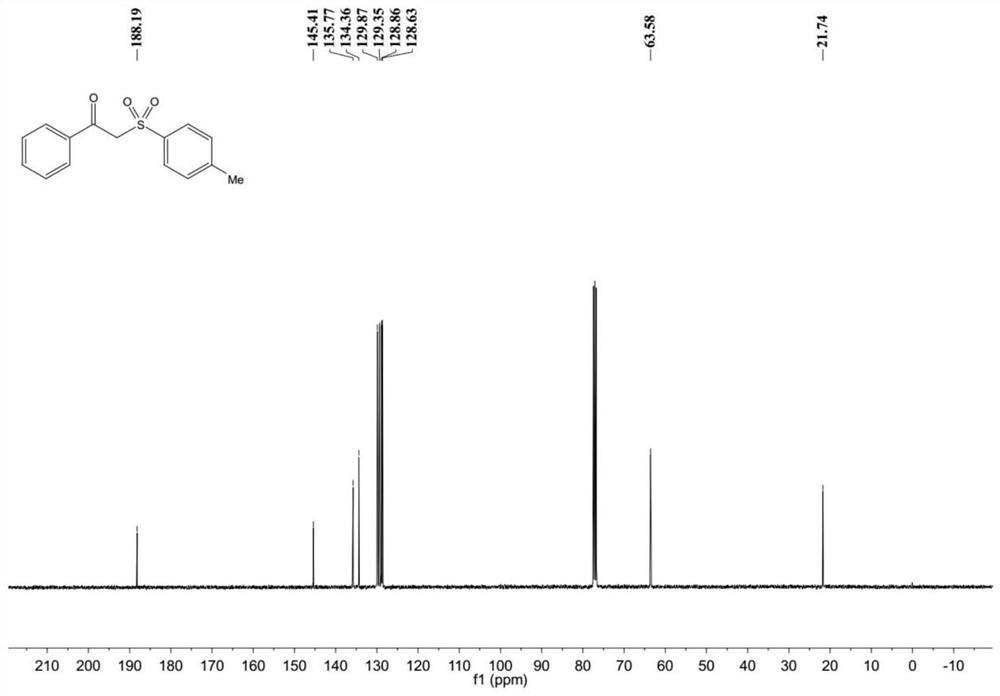
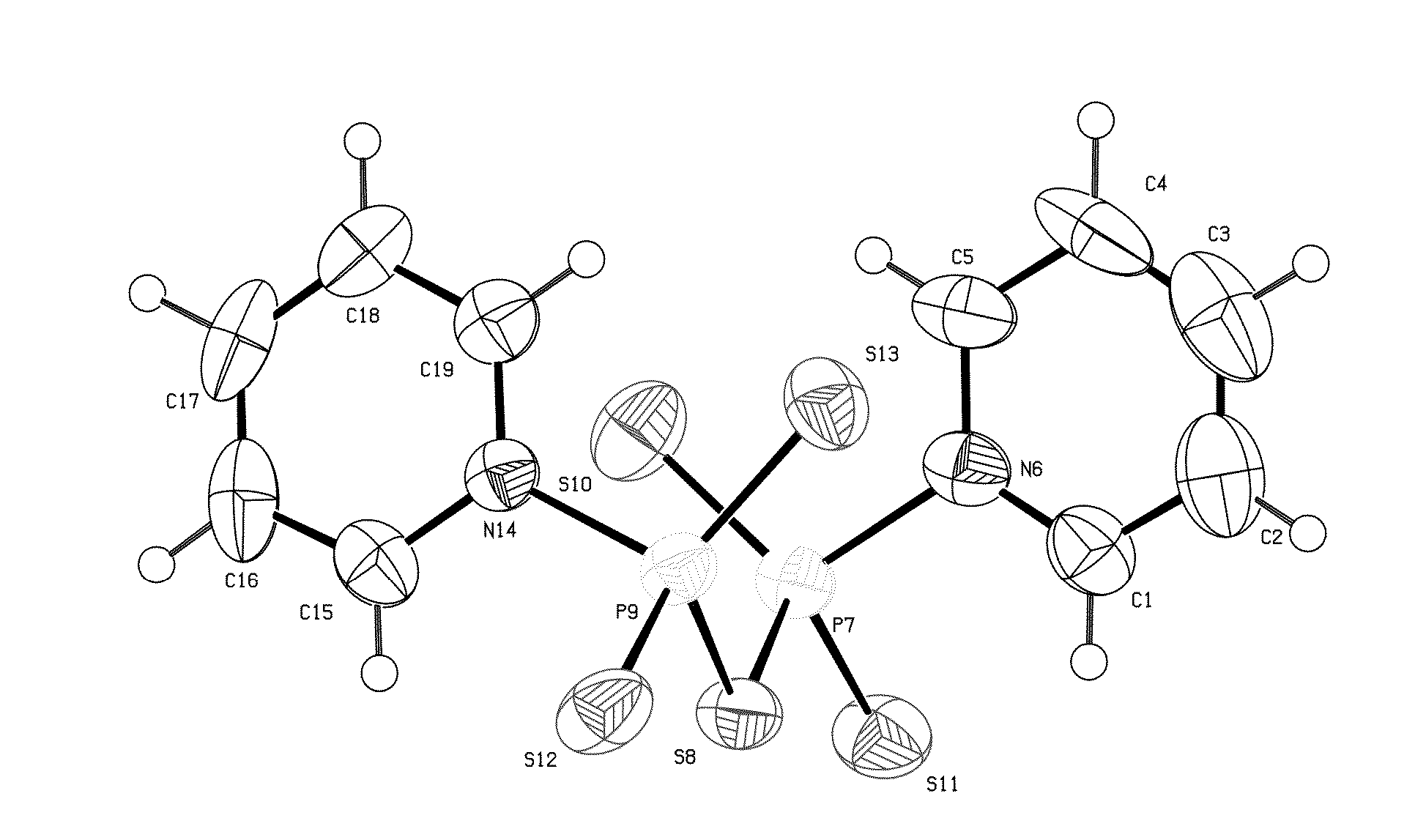
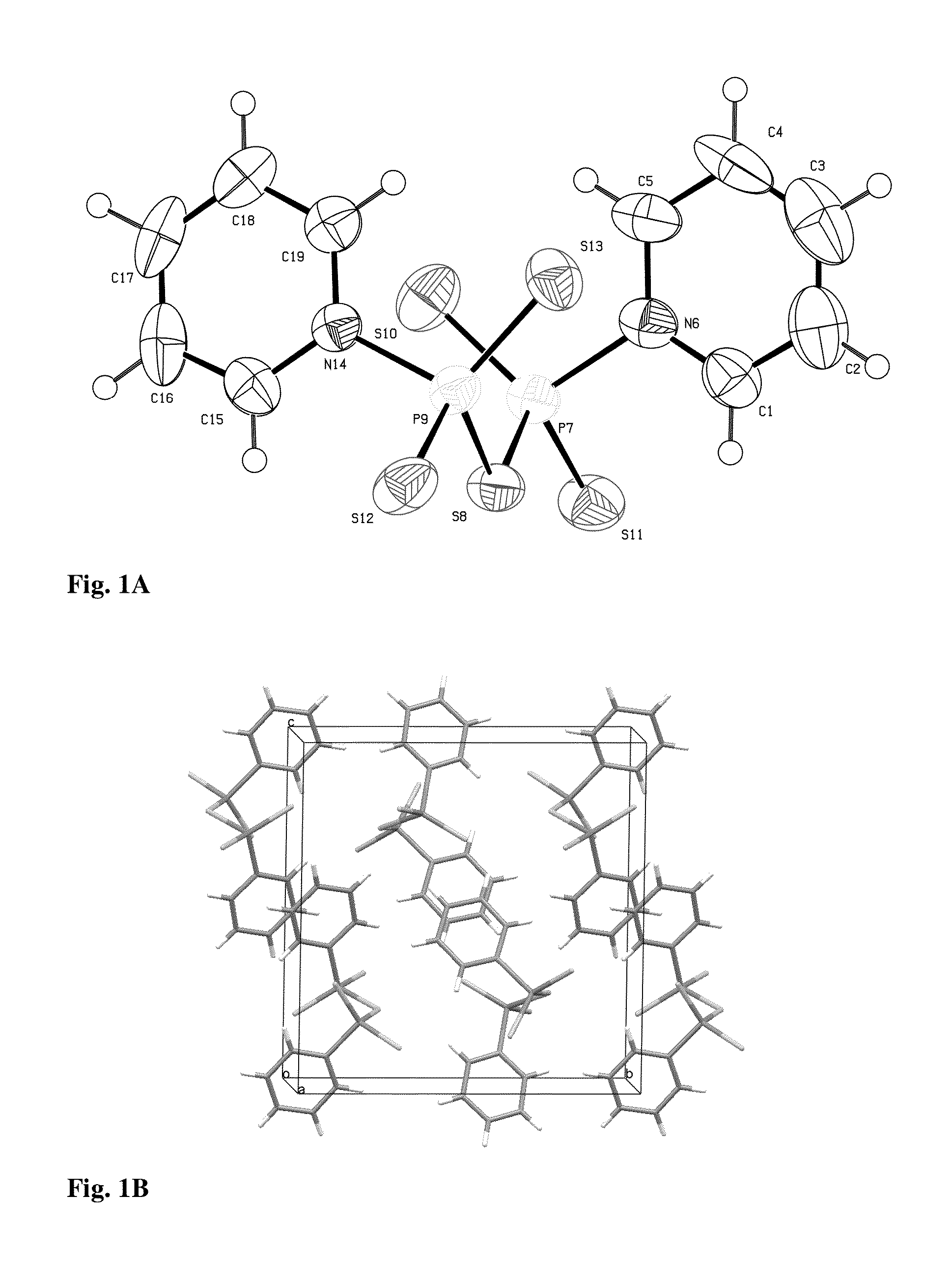
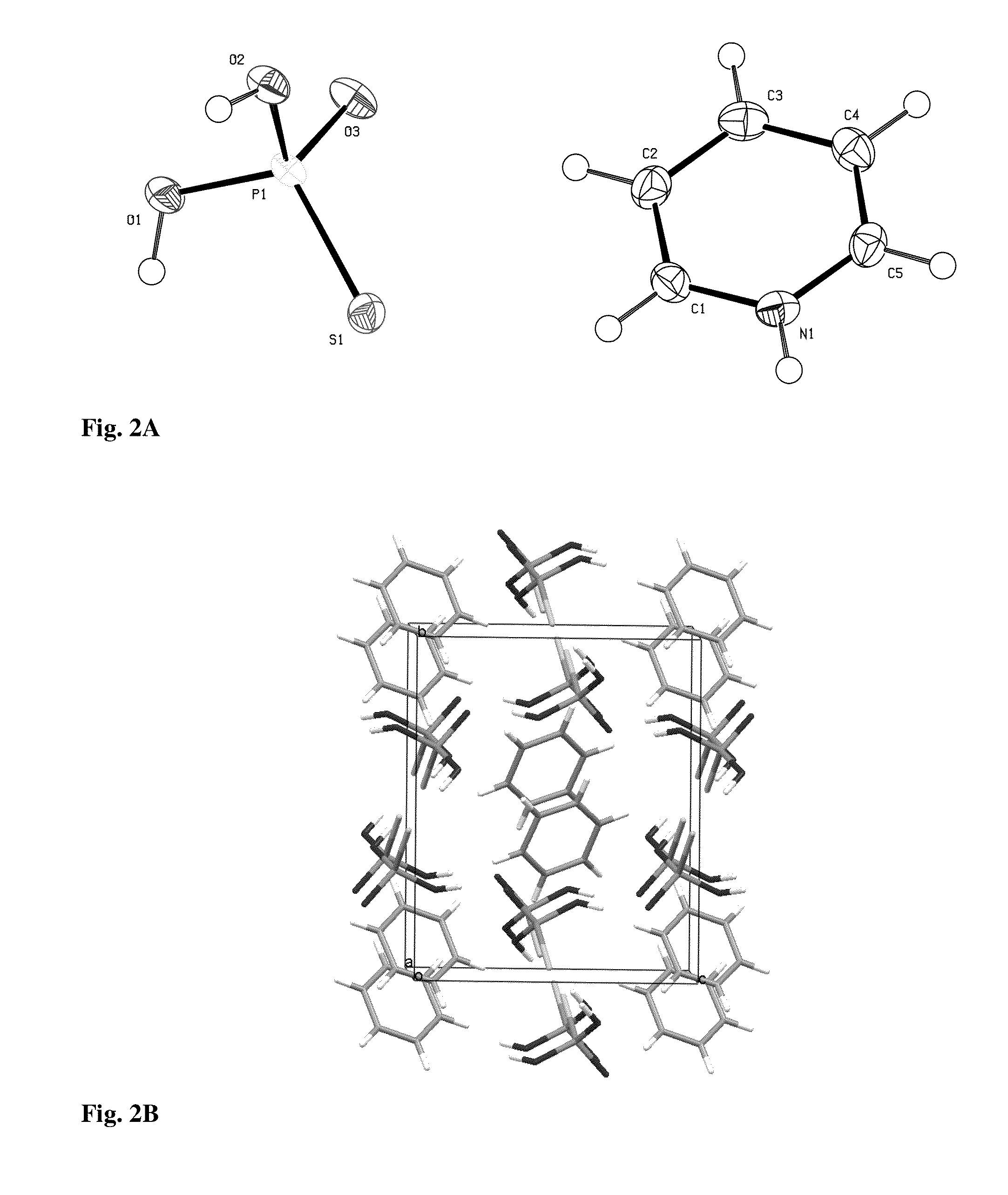
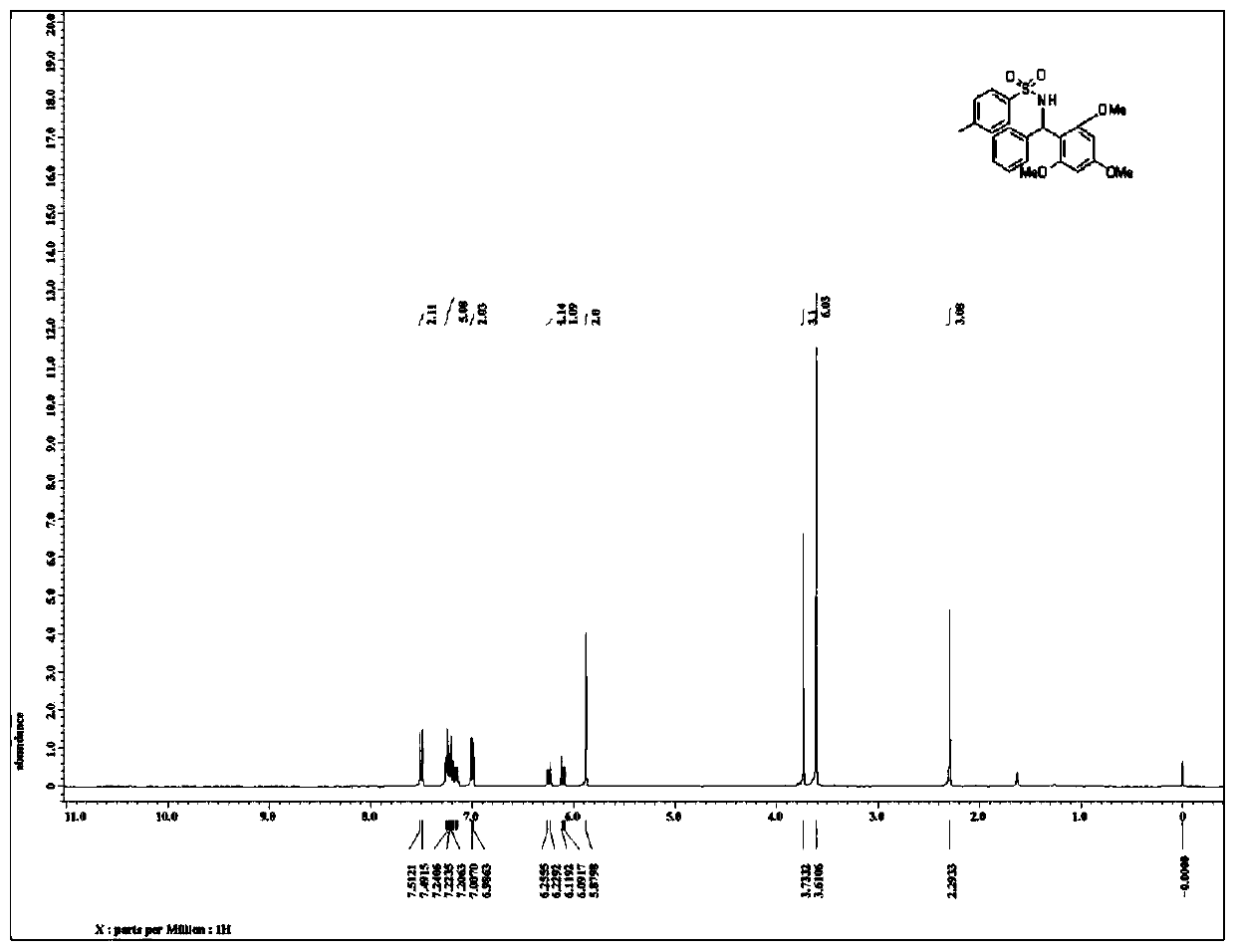
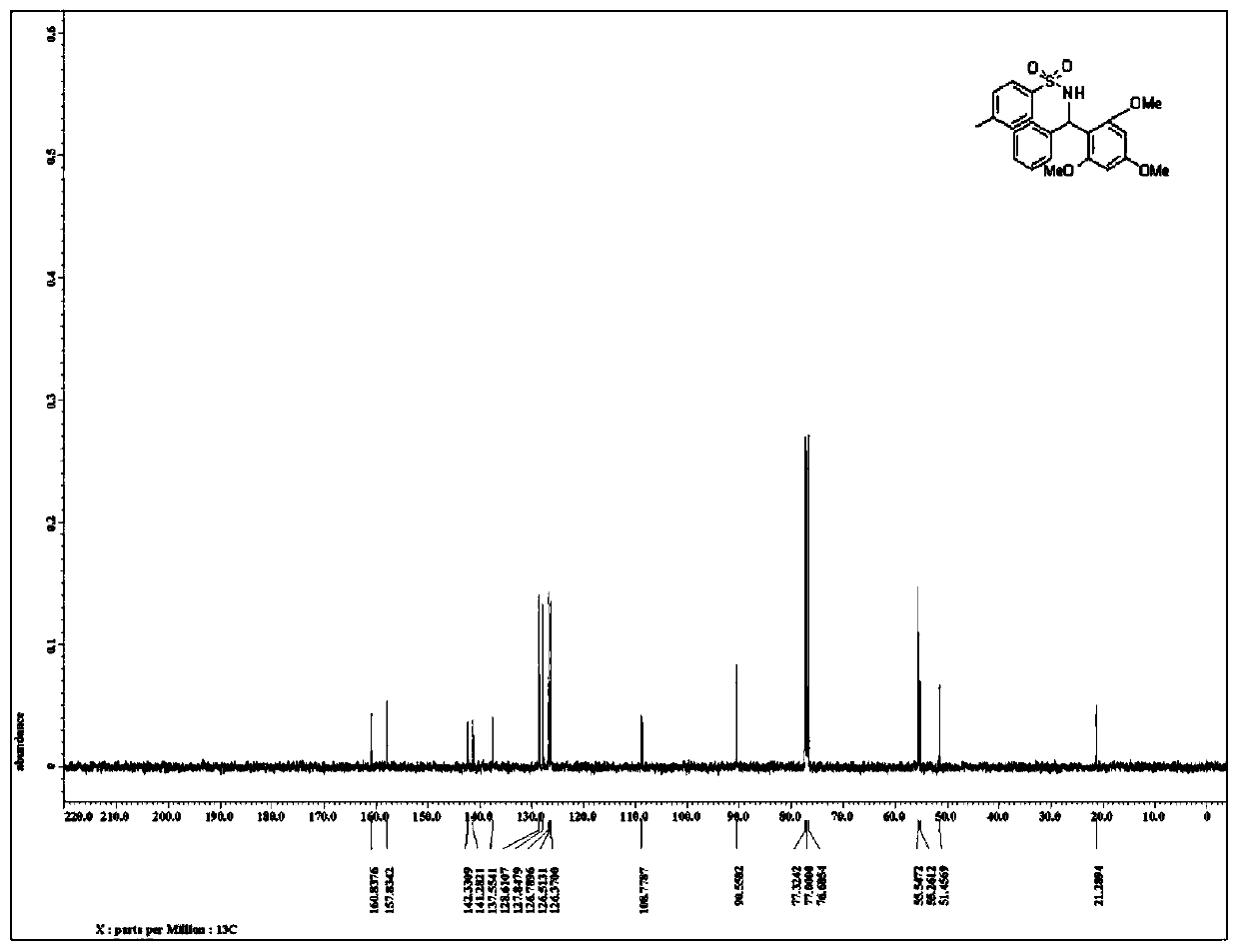
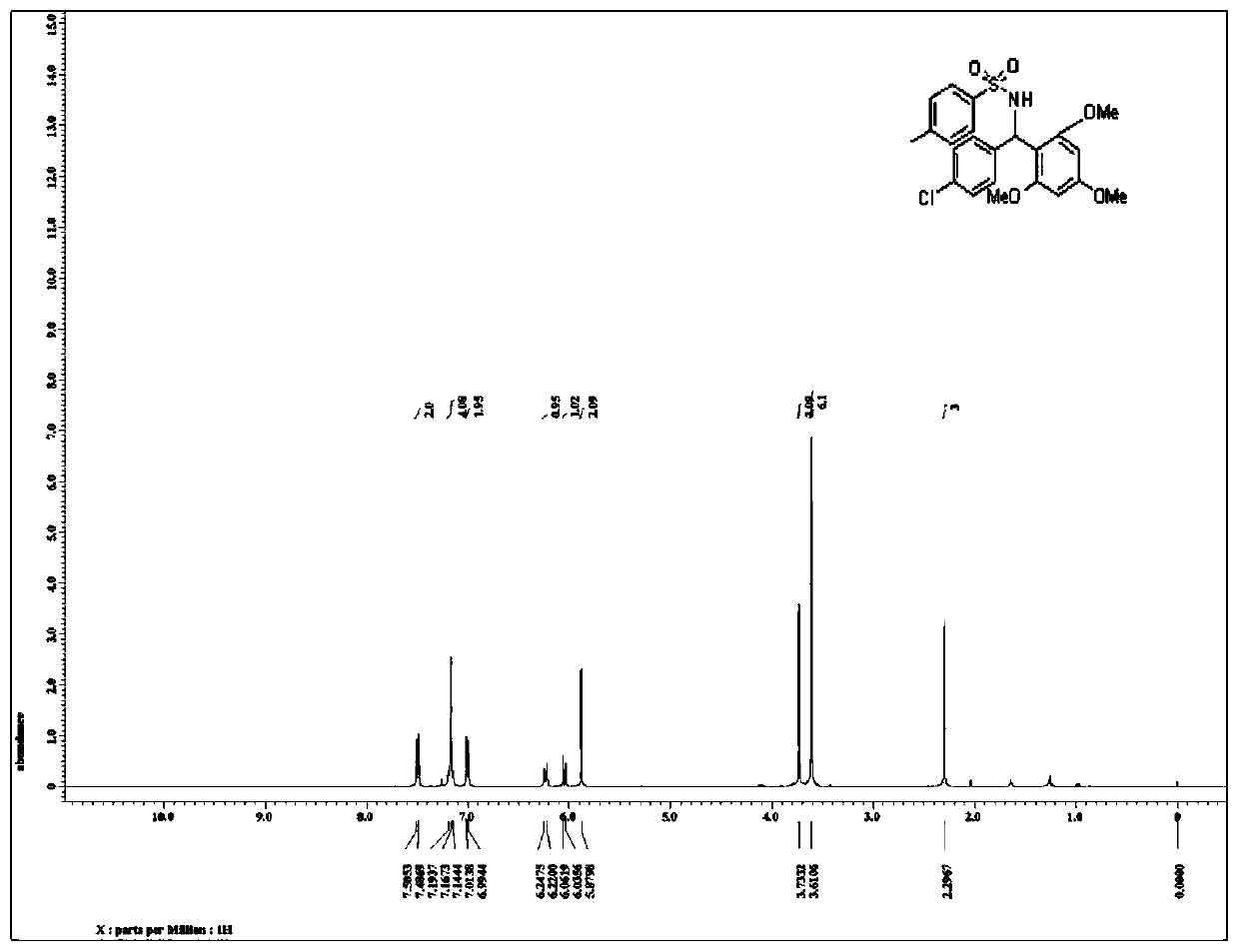
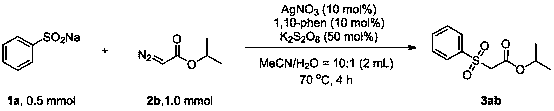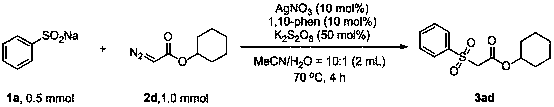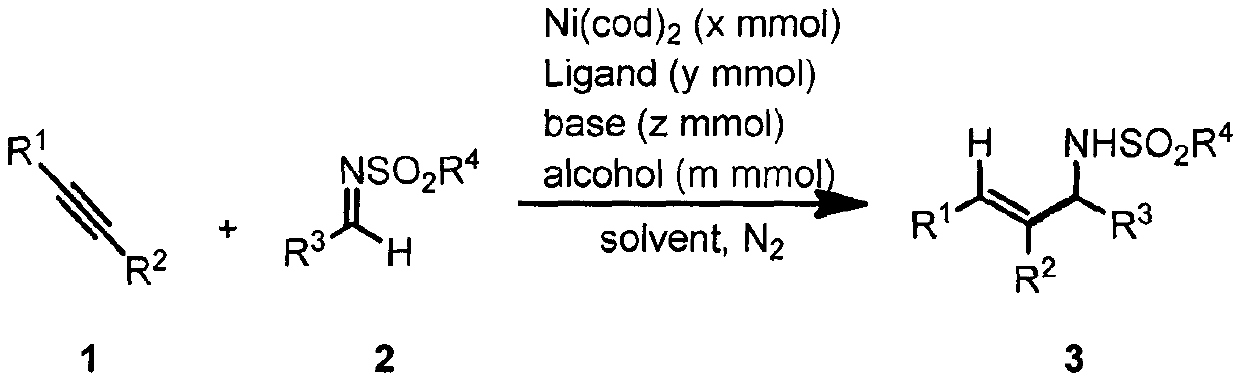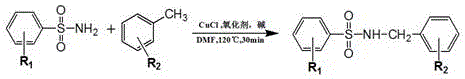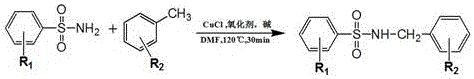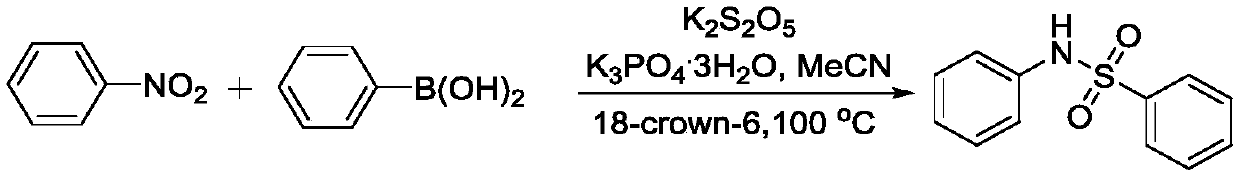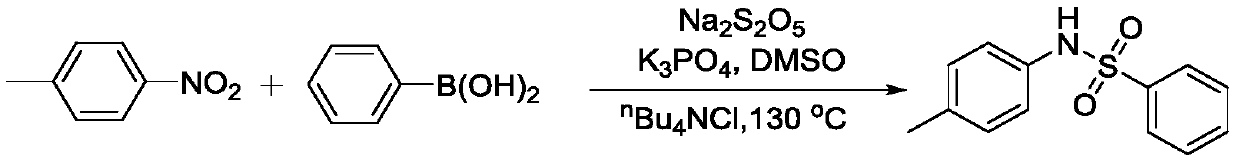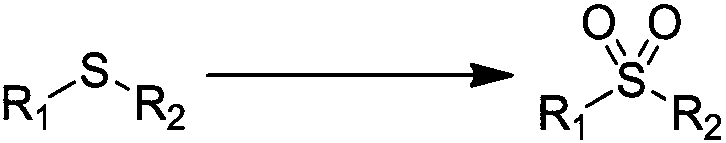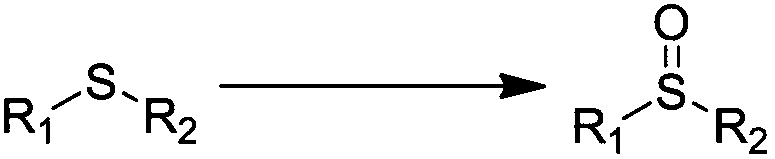Patents
Literature
58results about "Sulfonyl/sulfinyl group formation/introduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing sulfone or sulfoxide compound
InactiveUS6916938B2Good choiceAdvantageously producedSulfonyl/sulfinyl group formation/introductionOrganic compound preparationSulfoneSulfide
There is provided a method for preparing a sulfone or sulfoxide compound, characterized in that a sulfide compound is allowed to react with hydrogen peroxide in the presence of a metal oxide catalyst formed by the reaction of hydrogen peroxide with at least one metal or metal compound selected from tungsten metal; molybdenum metal; a tungsten compound comprising tungsten and a Group IIIb, IVb, Vb, or VIb element exclusive of oxygen; and a molybdenum compound comprising molybdenum and a Group IIIb, IVb, Vb, or VIb element exclusive of oxygen.
Owner:SUMITOMO CHEM CO LTD
Method for preparing sulfonamide and derivatives thereof
InactiveCN104496738AEasy to operateMild reaction conditionsSulfonyl/sulfinyl group formation/introductionSulfonic acid amide preparationMetal catalystIodine
The invention discloses a method for preparing sulfonamide and derivatives thereof. The method comprises the following step of in the absence of a metal catalyst and in the presence of iodine serving as a catalyst and an oxidant, reacting sodium sulfinate and derivatives of sodium sulfinate with amine and derivatives of amine or ammonia water to form corresponding sulfonamide and derivatives of sulfonamide. According to the method, by adopting the characteristics of mild reaction conditions and no addition of transition metals, alkali, ligand and additives, sulfonamide and derivatives thereof are efficiently prepared, and the method has the advantages of broad substrate range and universality, inexpensive raw materials and mild conditions, easiness for treatment and safety, so that the reaction has good practicability and wide application.
Owner:HUAQIAO UNIVERSITY
Sulfocompound selective catalytic oxidation reaction system in aqueous phase
InactiveCN105949018AReduce dosageGood choiceSulfonyl/sulfinyl group formation/introductionOrganic compound preparationRoom temperatureEthyl acetate
The invention provides a sulfocompound selective catalytic oxidation reaction system in an aqueous phase. A catalyst, a sulfocompound and 30% hydrogen peroxide are stirred for 1.5-2 hours under room temperature in the aqueous phase according to the molar ratio of the catalyst to the sulfocompound to the 30% hydrogen peroxide being 1 to 400 to 1200, wherein the conversion rate is greater than 97%, and the selectivity of the product namely sulphone is greater than 94%; the catalyst, the sulfocompound and the 30% hydrogen peroxide are stirred for 6 hours under the room temperature in the aqueous phase according to the molar ratio of the catalyst to the sulfocompound to the 30% hydrogen peroxide being 1 to 1666 to 1666, wherein the conversion rate is greater than 90%, and the selectivity of the product namely sulphoxide is greater than 80%. According to the reaction system disclosed by the invention, after the reaction is completed, extraction is performed with ethyl acetate, after an organic phase is separated, the catalyst dispersed in the aqueous phase can be directly used for the next catalytic reaction, and the catalytic activity, the conversion rate and the selectivity are all kept. The sulfocompound selective catalytic oxidation reaction system disclosed by the invention has the advantages that water is used as a solvent, the reaction condition is mild, the catalytic activity is high, the selectivity of products is good, the consumption of the catalyst is low, and the catalyst can be repeatedly used.
Owner:NANYANG NORMAL UNIV
Preparation method of sulfuryl fluoride compound
InactiveCN105198683AHigh yieldMild reaction conditionsSulfonyl/sulfinyl group formation/introductionSulfonic acid preparationChemical synthesisSulfohydrazide
The invention relates to a preparation method of a sulfuryl fluoride compound. A sulfonyl hydrazide compound and a fluoride reagent serve as reaction raw materials. The preparation method includes the steps that a, the sulfonyl hydrazide compound with the structure (I) and the fluoride reagent are dispersed in a solvent, wherein the structure (I) is shown in the specification; b; a mixture obtained from the step a is stirred and heated to obtain the sulfuryl fluoride compound with the structure (II), wherein the structure (II) is shown in the specification (II). Compared with existing related technologies in the chemical synthesis field, the method of preparing sulfuryl fluoride from sulfonyl hydrazide is achieved for the first time. In the method, no catalyst needs to be added, reaction conditions are moderate, good compatibility can be achieved for water and air, and large-scale production is easy to achieve. The experimental result indicates that the yield of the obtained sulfuryl fluoride compound can reach up to 98%.
Owner:XINYANG NORMAL UNIVERSITY
Method for selectively oxidizing sulfide
ActiveCN102515999AImprove conversion rateHigh selectivitySulfonyl/sulfinyl group formation/introductionOrganic compound preparationReaction temperatureNitride
The invention relates to a method for selectively oxidizing sulfide. A mesoporous carbon nitride material is taken as a catalyst; oxygen is taken as an oxidant; the mass ratio of the raw material sulfide to the catalyst is (2000:1) to (1:1); the reaction temperature is 0-250 DEG C; the reaction time is 4-24 hours; and the sulfide is selectively oxidized, thereby obtaining the corresponding sulphoxide compound. The technology of preparing the sulphoxide compound by selectively oxidizing the sulfide provided by the invention has the advantages that the process flow is short, the three wastes are few, the conversion rate of the raw material sulfide is high and the selectivity of the product sulphoxide is high; the cheap oxygen is taken as the oxidant, so that the production cost is lowered; and after the reaction is ended, the catalyst is recycled according to a simple filtering method.
Owner:ZHEJIANG UNIV
Microporous materials (TIQ-6 and METIQ-6) of high surface area active in oxidation reactions
InactiveUS6843978B2High activityHigh selectivityAluminium compoundsSulfonyl/sulfinyl group formation/introductionOxygenOrganic compound
The present invention refers to a microporous material formed by oxygen, silicon, germanium, aluminum, boron, gallium, zirconium and / or titanium in its composition, called TIQ-6, to its catalytic applications in oxidation reactions, and to a method of the TIQ-6 material's preparation based on the synthesis of a gel with a titanium and / or zirconium content, its hydrothermal treatment under controlled conditions, and the treatment of the resulting laminar material with a solution of an organic compound containing a proton accepting group. This swollen material is subjected to a specific treatment to obtain a high external area delaminated solid. A material, METIQ-6, similar to the TIQ-6 material, but also having organic groups anchored on its surface incorporated by a post-synthesis process onto the TIQ-6 material is also claimed.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC) +1
Methods for producing alkylbenzenes, paraffins, olefins and oxo alcohols from waste plastic feedstocks
ActiveUS20170044465A1Broad representationHydrocarbon by dehydrogenationSulfonyl/sulfinyl group formation/introductionOxo alcoholParaffin oils
The present invention relates generally to methods for producing detergent compounds from waste plastic feedstocks. More specifically, the invention relates to methods for producing detergent intermediates, including alkylbenzenes, paraffins, olefins, oxo alcohols, and surfactant derivatives thereof from waste plastic feedstock.
Owner:THE PROCTER & GAMBLE COMPANY
Aryl sulfonamide tertiary amine compound synthesizing method
ActiveCN107954906ARaw materials are easy to getThe synthesis process is simpleSulfonyl/sulfinyl group formation/introductionSulfonic acid amide preparationArylSulfonyl chloride
The invention provides an aryl sulfonamide tertiary amine compound synthesizing method. According to the method, in an anhydrous aprotic solvent and under nitrogen protection, acryl sulfonyl chlorideand di-tertiary amine react for 1 to 12 hours according to a molar ratio of (1 to 1) to (1 to 20) under the condition of 50 to 150 DEG C, and the aryl sulfonamide tertiary amine compound can be obtained by purification. According to the method disclosed by the invention, the acryl sulfonyl chloride and the di-tertiary amine can directly generate C-S bond formation in the aprotic solvent and generate C-N bond breakage at the same time. According to the one-step aryl sulfonamide synthesizing method, raw materials are easy to obtain, a synthesizing technology is simple, operation is convenient, cost is low, and yield is high.
Owner:山东中新科农生物科技有限公司
Preparation method of sulfonamides compound
ActiveCN107033106ASafe and stable process conditionsRaw materials are cheap and easy to getSulfonyl/sulfinyl group formation/introductionSulfonic acid amide preparationSynthesis methodsMetal catalyst
The invention discloses a reparation method of a sulfonamides compound. The preparation method includes: using thiophenol and amine which are simple and easy to get as raw materials; enabling the raw materials to be in direct oxidation coupling reaction under mediation of safe and stable iodine pentoxide to prepare sulfonamide. The preparation method has the advantages that reaction conditions are mild (60 DEG C), the raw materials are simple and easy to get and low in price, reaction environment is friendly, a substrate is wide in application range, and metal catalysts and harsh reaction conditions like low or high temperature and zero water and zero oxygen are not needed, so that metal pollution related to common synthesis methods is avoided; the preparation method further has the advantages of simple, convenient and safe operation, stable process condition and easiness in product purification and is suitable for large-scale production.
Owner:QUFU NORMAL UNIV
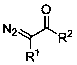
Method for preparing beta-carbonyl sulfone
ActiveCN110818600AImprove economySimple and fast operationSulfonyl/sulfinyl group formation/introductionOrganic compound preparationPotassium persulfatePtru catalyst
The invention discloses a method for preparing beta-carbonyl sulfone. The preparation method comprises the following steps: by taking an alpha-carbonyl diazo compound and sodium arylsulfinate as reaction substrates, cheap silver nitrate as an optimal catalyst, 1, 10-phenanthroline as a ligand and potassium persulfate as an oxidant, carrying out coupling reaction in a mixed solvent of acetonitrileand water to obtain the beta-carbonyl sulfone compound. Compared with the prior art, the method has the advantages of wide reaction substrate range, short reaction time, high reaction yield, mild reaction conditions and the like. Non-toxic and harmless reagents are used as reaction raw materials, so that the method is harmless to the environment and meets the requirements of modern green chemicaldevelopment. Post-reaction treatment is simple, and separation and purification are facilitated. In addition, the reaction can realize gram-scale synthesis, and lays a foundation for practical application.
Owner:SUZHOU UNIV
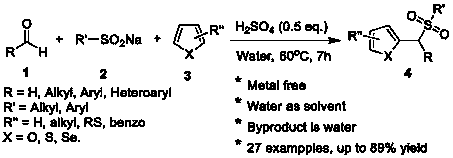
Synthesis method of aryl(chalcogen-heteroaryl)methyl sulfone
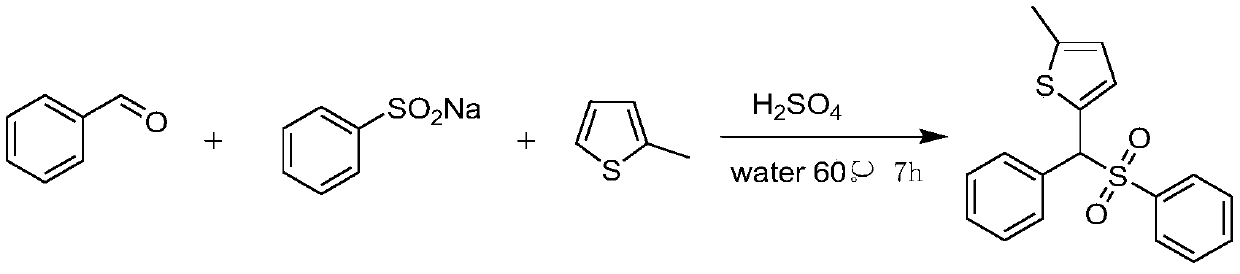
Sulfone is an important drug and bioactive compound. Sulfone is widely used as a medicament, such as a medicament gamma-secretase inhibitor for preventing Alzheimer's disease, and is widely applied tobioactive compounds, natural products and agricultural chemicals, such as currently popular herbicide mesotrione. Sulfone is also most often used as an intermediate for organic synthesis. On the other hand, a chalcogen heterocyclic stent has biological activity, such as antitumor drugs and antiproliferative drugs. As is well-known, thiophene, furan and selenium compounds have a variety of biological activities, such as anti-inflammatory agents, anti-HIV PR inhibitors, NQO2 inhibitors, and anticancer agents. The invention develops a simple, convenient and efficient Bronsted acid, i.e., sulfuric acid and catalyzed three components react in water to synthesize aryl(chalcogen-heteroaryl)methyl sulfone, the yield is good to be very high, and the substrate range is wide. The synthesis method isenvironment-friendly and economic, and does not need metal catalysis. The sulfone product can be effectively converted into bactericide analogues and aryl heteroaryl ketones.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Method for preparing diaryl sulfone
ActiveCN103641674AImprove practicalityHigh selectivitySulfonyl/sulfinyl group formation/introductionOrganic compound preparationArylAlcohol
The invention discloses a method for preparing diaryl sulfone and particularly relates to a copper chloride promoted method for preparing diaryl sulfone. The method is characterized by comprising the following step: taking aryl sulfinate as a raw material, carrying out a desulfurization coupling reaction on aryl sulfinate in an alcohol solvent under the action of copper chloride, so as to synthesize diaryl sulfone. According to the method, any ligand is not needed, inert gas shielding is not needed, and the diaryl sulfone of which the yield is 96 percent can be obtained.
Owner:ZHEJIANG ZHONGXIN FLUORIDE MATERIALS CO LTD
Copper catalytic synthesis method of organic sulphone compound and application
InactiveCN108129478AReduce pollutionImprove compatibilitySulfonyl/sulfinyl group formation/introductionPtru catalystOrganic synthesis
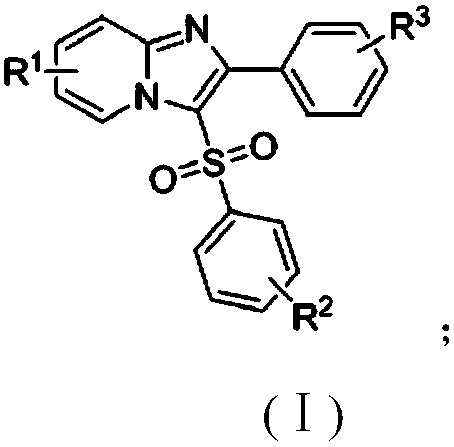
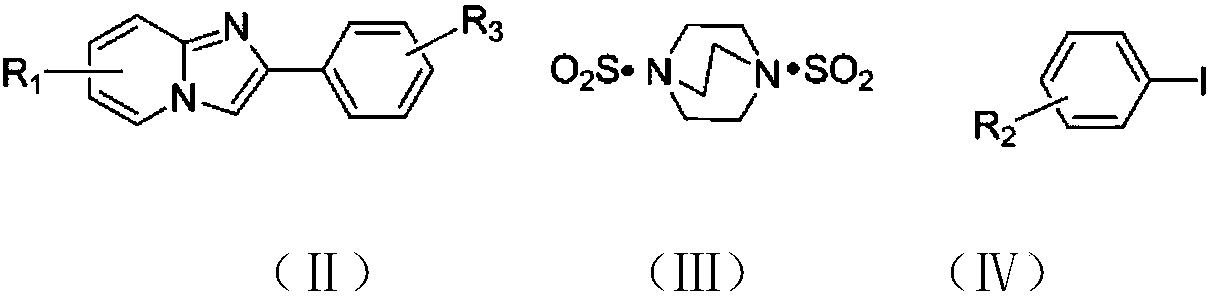
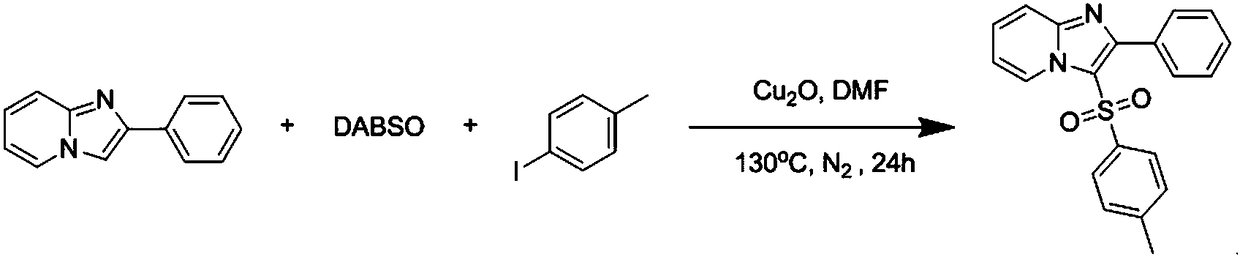
The invention discloses a copper catalytic synthesis method of an organic sulphone compound and application, and relates to the field of organic synthesis. An imidazol[1,2-a] pyridine compound, a halogenated aromatics compound and DABSO are adopted as raw materials, DMF is adopted as a solvent, monovalent copper is adopted as a catalyst, the reaction temperature is 110 DEG C to 150 DEG C, and a C-3-sulfone-containing imidazol[1,2-a] pyridine compound is high-efficiently synthesized. Compared with a traditional synthesis method, the method has the advantages of mild reaction condition, low cost, little environmental pollution, high yield, good functional group compatibility, convenience in separation and purification and the like.
Owner:QUFU NORMAL UNIV
Method for promoting reductive coupling reaction of imine and alkyne by means of alcohol to build allyl amine derivative
InactiveCN110229085AWide variety of sourcesLow priceSulfonyl/sulfinyl group formation/introductionSulfonic acid amide preparationElectronegativityCarbene
The invention relates to a method for using alcohol as a green cheap reduction agent and carrying out an intermolecular reductive coupling reaction on ordinary imine and ordinary alkyne under catalysis of cheap transition metal to efficiently produce a polysubstituted allyl amine derivative. The method is economic and practical. The critical points of the method for solving problems lie in the facts that a large-steric hindrance high-electronegativity carbene ligand is found to improve the reaction activity, and meanwhile, by designing a chiral carbene ligand, chiral control of part of substrates is achieved; and the cheap metal nickle and the green reduction agent isopropyl alcohol are used jointly, so that the method is economic, practical and environmentally friendly, and is in accord with principles of sustainable development.
Owner:NANKAI UNIV
Synthesis process of sulfonamide compounds in microwave system
ActiveCN106631915ASimple and fast operationHigh yieldSulfonyl/sulfinyl group formation/introductionSulfonic acid amide preparationEnvironmental resistanceEthyl acetate
The invention discloses a synthesis process of sulfonamide compounds in a microwave system. The synthesis process is realized by the following steps: by using CuCl as a catalyst and FeCl3 as an oxidant, carrying out carbon-hydrogen activating and carbon-nitrogen coupling reaction in a DMF (Dimethyl Formamide) by substituting sulfanilamide and methylbenzene through microwave heating and efficient catalysis for 10 to 60 minutes; extracting a product by using ethyl acetate; carrying out vacuum concentration; carrying out column chromatographic purification on a product to obtain the sulfonamide compounds. The synthesis process is a method for efficiently preparing the sulfonamide compounds, which is environment-friendly and is simple and convenient to operate. Compared with the prior art, the synthetic process disclosed by the invention has the advantages of remarkably-increased reaction speed compared with that under a conventional heating condition, mild reaction conditions, simple operation, high yield, safety, low cost and environmental protection.
Owner:FUJIAN MEDICAL UNIV
Methods for producing alkylbenzenes, paraffins, olefins and oxo alcohols from waste plastic feedstocks
ActiveUS10308896B2Broad representationHydrocarbon by dehydrogenationSulfonyl/sulfinyl group formation/introductionOxo alcoholAlkylbenzenes
The present invention relates generally to methods for producing detergent compounds from waste plastic feedstocks. More specifically, the invention relates to methods for producing detergent intermediates, including alkylbenzenes, paraffins, olefins, oxo alcohols, and surfactant derivatives thereof from waste plastic feedstock.
Owner:PROCTER & GAMBLE CO
Method for preparing beta-ketosulfone compound through visible light mediated atopic acid decarboxylation ketonization reaction
ActiveCN110981676ARaw materials are easy to getEasy to operateSulfonyl/sulfinyl group formation/introductionOrganic compound preparationSulfohydrazidePhotochemistry
The invention relates to a method for preparing a beta-ketosulfone compound through a visible light mediated atopic acid decarboxylation ketonization reaction, and belongs to the field of compound preparation. According to the method disclosed by the invention, environment-friendly atortic acid with wide sources and stable odorless sulfonyl hydrazine are used as reactants, fluorescein, inorganic base and potassium iodide are added and then fully dissolved in a mixed solvent of acetonitrile and water, a reaction is carried out in an oxygen atmosphere (communicated with oxygen spheres), and thebeta-ketosulfone compound can be obtained through the reaction under visible light irradiation. The preparation method disclosed by the invention has the characteristics of simple and easily availableraw materials, simple operation, mild reaction conditions, no metal residue and insensitivity of a reaction system to water.
Owner:SOUTHWEST UNIVERSITY
Continuous flow carboxylation reaction
ActiveUS9725413B2Sulfonyl/sulfinyl group formation/introductionChemical/physical/physico-chemical stationary reactorsArylContinuous flow
The present invention is related to a two-step carboxylation reaction of an aryl group using continuous flow reaction conditions. This process permits large scale synthesis of useful reaction products in high yield.
Owner:NOVARTIS AG
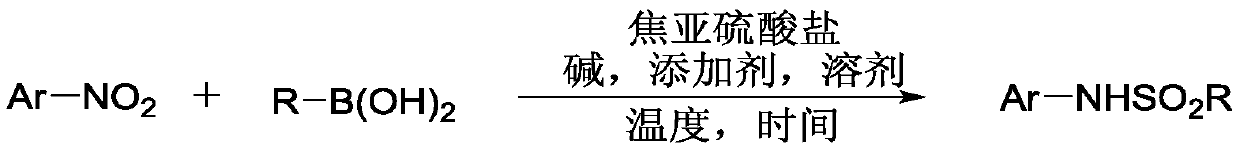
Method for coupling nitroaromatic compound and boric acid compound to synthesize sulfonamide compound
ActiveCN110372463AEasy to operateMild reaction conditionsSulfonyl/sulfinyl group formation/introductionSulfonic acid amide preparationOrganic solventOrganic synthesis
The invention belongs to the field of organic synthesis, and specifically discloses a method for coupling a nitroaromatic compound and a boric acid compound to synthesize a sulfonamide compound. The method for coupling the nitroaromatic compound and the boric acid compound to synthesize the sulfonamide compound comprises the steps that in an organic solvent, pyrosulfite is used as a source of SO2,and heating is carried out for a coupling reaction, and then after the post-treatment, the sulfonamide compound is obtained. The method for coupling the nitroaromatic compound and the boric acid compound to synthesize the sulfonamide compound is simple in operation, does not require nitrogen protection, and can be carried out under air. The nitroaromatic compound and the boric acid compound are abundant in source, relatively low in price, high in reaction yield, wide in applicability of a substrate and free in metal residual. The method for coupling the nitroaromatic compound and the boric acid compound to synthesize the sulfonamide compound can be used for synthesizing a series of sulfonamide compounds, and the synthesized compounds have wide application value in the fields of pesticidesand medicines.
Owner:ZHEJIANG UNIV
Application of HEH in catalyzing reaction of aryl halogen and aryl sulfinate to prepare sulfone compounds
ActiveCN111068776AEnables cross-coupling reactionsHigh yieldSulfonyl/sulfinyl group formation/introductionOrganic compound preparationPtru catalystPhotochemistry
The invention discloses application of 2,6-dimethyl-1,4-dihydro-3,5-pyridine diethyl dicarboxylate as a visible light reduction catalyst to induce transition-metal-free catalysis of aryl halogen and aryl sulfinate so as to prepare sulfone compounds. The method comprises the following steps: under inert gas protection, adding the reactants into a reaction container provided with a stirring device according to the molar ratio of aryl halogen compounds, sulfinate compounds, inorganic alkali to HEH being 1:2:1.5:0.2, then adding dimethyl sulfoxide, and carrying out stirring reaction for 24 hours at room temperature under blue LED irradiation to obtain the sulfone compounds. According to the method, the HEH is used as a catalyst for the first time under the condition that no auxiliary transition metal catalyst is added, and a series of cross-coupling reactions of aryl halogen and sulfinate are realized. In addition, the whole process is green, efficient and easy to operate, and the method is a good method for synthesizing sulfone compounds.
Owner:SUZHOU UNIV
Microporous materials (TIQ-6 and METIQ-6) of high surface area active in oxidation reactions
InactiveUS20020193239A1High activityHigh selectivityAluminium compoundsSulfonyl/sulfinyl group formation/introductionOxygenOrganic compound
The present invention refers to a microporous material formed by oxygen, silicon, germanium, aluminum, boron, gallium, zirconium and / or titanium in its composition, called TIQ-6, to the process for preparing it and to its catalytic applications in oxidation reactions. The TIQ-6 material's preparation method is based on the synthesis of a gel with a titanium and / or zirconium content, its hydrothermal treatment under controlled conditions, and the treatment of the resulting laminar material with a solution of an organic compound containing a proton accepting group. This swollen material is subjected to a specific treatment to obtain a high external area delaminated solid. A material, METIQ-6, similar to the TIQ-6 material, but also having organic groups anchored on its surface incorporated by a post-synthesis process onto the TIQ-6 material is also claimed.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC) +1
Synthesis method of 2-cyanoalkylsulfonyl 3,4-dihydronaphthalene compound
ActiveCN111039737AHigh yieldConvenient sourceSulfonyl/sulfinyl group formation/introductionOrganic compound preparationAcyl groupOxime
The invention discloses a synthesis strategy for constructing a 2-cyanoalkyl sulfonyl substituted 3,4-dihydronaphthalene compound by taking MCPs, a cyclobutanone oxime ester compound and K2S2O5 as rawmaterials through visible light catalysis, wherein two carbon-carbon sigma bonds are broken in a one-pot reaction to form a carbon-carbon bond and two carbon-sulfur bonds. According to the invention,cyanoalkyl free radicals are formed to capture SO2, and subsequently sulfonyl free radicals are formed to carry out ring-opening and cyclizing on MCPs; and the synthesis method disclosed by the invention has the advantages of mild reaction conditions, simplicity, high efficiency, easily available raw material sources, wide substrate application range and high target product yield.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
Method of preparing chiral amine by catalytic hydrogenation of N- ulfimide by asymmetrical nickel
ActiveCN109942506AEfficient preparationMild conditionsSulfonyl/sulfinyl group formation/introductionSulfonic acid amide preparationHydrogen pressureAsymmetric hydrogenation
The invention discloses a method of preparing chiral amine by catalytic hydrogenation of N- sulfimide by asymmetrical nickel. The method comprises the following step: in a solvent, hydrogenating N-sulfimide shown in a formula (1) to a chiral amine compound shown in a formula (2) by catalysis of a chiral catalyst of nickel at a certain hydrogen pressure and temperature, wherein the structural formulae of the formulae (1) and (2) as shown in the description, The reaction method is mild in condition and simple to operate, can achieve good reaction yield and reaction efficiency, and has a relatively good application effect.
Owner:SHANGHAI JIAO TONG UNIV
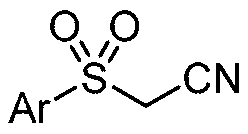
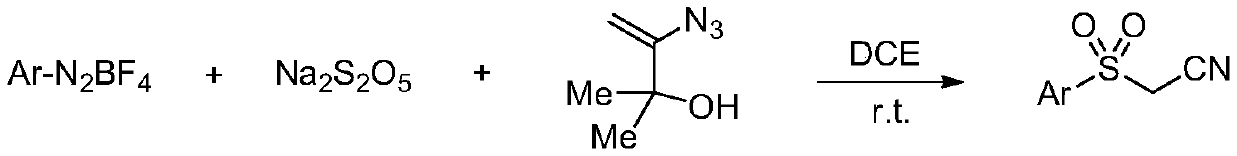
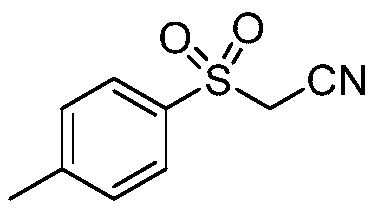
Preparation method of sulfonyl acetonitrile compound
ActiveCN111517904AEfficient constructionReduce usageSulfonyl/sulfinyl group formation/introductionOrganic compound preparationPtru catalystAcyl group
The invention belongs to the technical field of organic chemistry, and particularly relates to a preparation method of sulfonyl acetonitrile compounds. The structure of the compound is characterized by 1H NMR, 13C NMR and other methods and is confirmed. The method comprises the following steps: taking 2, 2-dichloroethane as a solvent and aryl diazonium salt and sodium pyrosulfite (Na2S2O5) as a catalyst without metal catalyst, generating aryl sulfonyl free radicals on site at room temperature, carrying out free radical addition reaction on 3-azido-2-methylbutyl-3-ene-2-ol, and removing monomolecular nitrogen and 2-hydroxypropyl free radicals to obtain the sulfonyl acetonitrile compound. The preparation method of the sulfonyl acetonitrile compound has the advantages of being mild in condition, simple, efficient, high in reaction yield, wide in substrate application range, good in product purity, convenient to separate and purify and good in application value.
Owner:TAIZHOU UNIV
Preparation method of beta-carbonyl sulfone compound
ActiveCN111978216AImprove toleranceSimple and fast operationSulfonyl/sulfinyl group formation/introductionOrganic compound preparationOrganic solventPtru catalyst
The invention discloses a preparation method of a beta-carbonyl sulfone compound, and belongs to the technical field of organic synthesis. The preparation method of the beta-carbonyl sulfone compoundis provided for solving the problems that in the prior art, operation is complex, the substrate range is narrow, and the functional group tolerance is poor. The preparation method comprises the stepsof taking a compound shown in formula I and a compound shown in formula II as raw materials, taking copper salt as a catalyst, carrying out reaction in an organic solvent, and after the reaction is completed, carrying out aftertreatment to obtain the beta-carbonyl sulfone compound. The preparation method is simple and convenient to operate, mild in reaction condition, wide in substrate range and good in functional group tolerance, the yield reaches up to 93%, and the synthesis cost is remarkably reduced.
Owner:SOUTHWEST MEDICAL UNIVERISTY
Thionation process and a thionating agent
ActiveUS20130102774A1Easy to separateSulfonyl/sulfinyl group formation/introductionPhosphorus sulfur/selenium/tellurium compoundsMedicinal chemistryCrystallization
A process for transforming a group >C═O (I) in a compound into a group >C═S (II) or into a tautomeric form of group (II) in a reaction giving a thionated reaction product, by use of crystalline P2S5.2 C5H5N as a thionating agent. A thionating agent which is crystalline P2S5.2 C5H5N.
Owner:VIRONOVA THIONATION
Synthesis method of N-diarylmethyl sulfonamide compound
ActiveCN111170899AEfficient synthesisBroad biological activitySulfonyl/sulfinyl group formation/introductionSulfonic acid amide preparationOrganic synthesisEthyl acetate
The invention discloses a synthesis method of an N-diarylmethyl sulfonamide compound, and belongs to the technical field of organic synthesis intermediates. According to the synthesis method, the N-diarylmethyl sulfonamide compound is synthesized by utilizing a cascade reaction; the method specifically comprises the following steps: (1) adding an aromatic aldehyde, a sulfonamide, trimethoxybenzeneor a derivative thereof, and Lewis acid into a reaction tube according to a molar ratio of 1.0: 1.0: 1.0: 0.1, and carrying out magnetic stirring reaction at room temperature for 8-24 hours by taking1, 2-dichloroethane as a solvent; and (2) after the reaction is finished, evaporating under reduced pressure to remove most of the solvent, and carrying out column chromatography separation and purification on a residual mixed solution by using petroleum ether and ethyl acetate in a volume ratio of (1: 1)-(2: 1) as leacheate to obtain the product. The N-diarylmethyl sulfonamide compound synthesized by the method has wide application in the fields of medicines, pesticides and the like. The synthesis method disclosed by the invention has the advantages of low cost, simplicity in operation, highyield, mild conditions and the like, and has a good application prospect.
Owner:内蒙古赤峰市柏善医药有限公司
Chiral sulfonamide derivative and preparation method and application thereof
ActiveCN109134402AEasy to operateMild reaction conditionsSulfonyl/sulfinyl group formation/introductionSulfonic acid amide preparationMolecular sieveAryl
Owner:SUN YAT SEN UNIV
A reaction system for selective catalytic oxidation of sulfur-containing compounds in aqueous phase
InactiveCN105949018BReduce dosageGood choiceSulfonyl/sulfinyl group formation/introductionOrganic compound preparationAcetic acidSulfur
The invention provides a sulfocompound selective catalytic oxidation reaction system in an aqueous phase. A catalyst, a sulfocompound and 30% hydrogen peroxide are stirred for 1.5-2 hours under room temperature in the aqueous phase according to the molar ratio of the catalyst to the sulfocompound to the 30% hydrogen peroxide being 1 to 400 to 1200, wherein the conversion rate is greater than 97%, and the selectivity of the product namely sulphone is greater than 94%; the catalyst, the sulfocompound and the 30% hydrogen peroxide are stirred for 6 hours under the room temperature in the aqueous phase according to the molar ratio of the catalyst to the sulfocompound to the 30% hydrogen peroxide being 1 to 1666 to 1666, wherein the conversion rate is greater than 90%, and the selectivity of the product namely sulphoxide is greater than 80%. According to the reaction system disclosed by the invention, after the reaction is completed, extraction is performed with ethyl acetate, after an organic phase is separated, the catalyst dispersed in the aqueous phase can be directly used for the next catalytic reaction, and the catalytic activity, the conversion rate and the selectivity are all kept. The sulfocompound selective catalytic oxidation reaction system disclosed by the invention has the advantages that water is used as a solvent, the reaction condition is mild, the catalytic activity is high, the selectivity of products is good, the consumption of the catalyst is low, and the catalyst can be repeatedly used.
Owner:NANYANG NORMAL UNIV
Method for preparing chiral amine by asymmetric nickel-catalyzed hydrogenation of n-sulfonimide
ActiveCN109942506BEfficient preparationMild conditionsSulfonyl/sulfinyl group formation/introductionSulfonic acid amide preparationImidePtru catalyst
The invention discloses a method of preparing chiral amine by catalytic hydrogenation of N- sulfimide by asymmetrical nickel. The method comprises the following step: in a solvent, hydrogenating N-sulfimide shown in a formula (1) to a chiral amine compound shown in a formula (2) by catalysis of a chiral catalyst of nickel at a certain hydrogen pressure and temperature, wherein the structural formulae of the formulae (1) and (2) as shown in the description, The reaction method is mild in condition and simple to operate, can achieve good reaction yield and reaction efficiency, and has a relatively good application effect.
Owner:SHANGHAI JIAOTONG UNIV
Popular searches
Organic-compounds/hydrides/coordination-complexes catalysts Chemical recycling Group 8/9/10/18 element organic compounds Molecular-sieve silicates Molecular sieve catalysts Hydroxy group formation/introduction Catalyst activation/preparation Carbonyl group formation/introduction Catalytic reactions Carbonyl compound preparation by oxidation
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com