Aryl sulfonamide tertiary amine compound synthesizing method
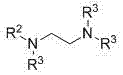
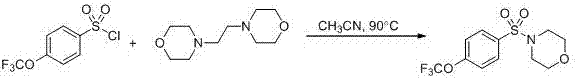
A technology of arylsulfonyl tertiary amide and synthesis method, which is applied in the preparation of sulfonic acid amide, the formation/introduction of sulfonyl group/sulfinyl group, organic chemistry, etc., can solve the problem of large-scale preparation and many reaction steps that are not suitable for industrialization. problem, to achieve the effect of low cost, convenient operation and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
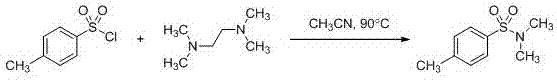
[0022] Embodiment 1, the synthesis of N,N-dimethyl-4-methylbenzenesulfonamide
[0023] Use a flame-dried 50ml two-neck flask, replace the nitrogen three times, use a syringe to dissolve p-toluenesulfonyl chloride (190mg, 1mmol) in acetonitrile (3ml) into the cooled reaction flask, heat the acetonitrile solution to reflux Finally, dissolve N,N,N,N-tetramethylethylenediamine (139mg, 1.2mmol) in 3ml of anhydrous acetonitrile, and slowly add it dropwise to the reaction bottle filled with p-toluenesulfonyl chloride with a syringe , then reacted for 1 hour under acetonitrile reflux, removed the heating, evaporated the solvent under reduced pressure, and separated by flash column chromatography to obtain 183 mg of the product, with a yield of 92%. Its synthetic formula is as follows:
[0024]
[0025] Spectral data: 1 H NMR (600 MHz, CDCl 3 ) δ (ppm): 7.66 (d, J = 8.2 Hz, 2H), 7.33(d, J = 8.0 Hz, 2H), 2.68 (s, 6H), 2.43 (s, 3H); 13 C NMR (151 MHz, CDCl 3 ) δ (ppm): 143.4,...
Embodiment 2
[0026] Embodiment 2, the synthesis of N, N-diethyl-p-toluenesulfonamide
[0027] Use a flame-dried 50ml two-neck flask, replace the nitrogen three times, use a syringe to dissolve p-toluenesulfonyl chloride (190mg, 1mmol) in acetonitrile (3ml) into the cooled reaction flask, heat the acetonitrile solution to reflux Finally, dissolve N,N,N,N-tetraethylethylenediamine (206mg, 1.2mmol) in 3ml of anhydrous acetonitrile, and slowly add it dropwise to the reaction bottle containing p-toluenesulfonyl chloride with a syringe , then reacted for 1 hour under acetonitrile reflux, removed the heat, evaporated the solvent under reduced pressure, and separated by flash column chromatography to obtain 197 mg of the product with a yield of 87%. Its synthetic formula is as follows:
[0028]
[0029] Spectral data: 1 H NMR (600 MHz, CDCl 3 ) δ (ppm): 7.68 (d, J = 8.2 Hz, 2H), 7.27(d, J = 8.0 Hz, 2H), 3.21 (q, J = 7.2 Hz, 4H), 2.41 (s, 3H), 1.11 (t, J = 7.1Hz, 6H); 13 C NMR (151 M...
Embodiment 3
[0030] Embodiment 3, N, the synthesis of N-dipropyl p-toluenesulfonamide
[0031] Use a flame-dried 50ml two-neck flask, replace nitrogen three times, use a syringe to dissolve p-toluenesulfonyl chloride (190mg, 1mmol) in acetonitrile (3ml) into the cooled reaction flask, heat the acetonitrile solution to reflux Finally, dissolve N,N,N,N-tetraisobutylethylenediamine (273mg, 1.2mmol) in 3ml of anhydrous acetonitrile, and slowly add it dropwise to the reaction mixture containing p-toluenesulfonyl chloride bottle, then reacted for 1 hour under acetonitrile reflux, removed the heat, evaporated the solvent under reduced pressure, and separated by flash column chromatography to obtain 216 mg of the product, with a yield of 85%. Its synthetic formula is as follows:
[0032]
[0033] Spectral data: 1 H NMR (600 MHz, CDCl 3 ) δ (ppm): 7.68 (d, J = 8.3 Hz, 2H), 7.27(d, J = 16.4 Hz, 2H), 3.05 (dd, J= 8.5, 6.9 Hz, 4H), 2.41 (s, 3H), 1.59 –1.49 (m, 4H), 0.86 (t, J = 7.4 Hz, 6H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com