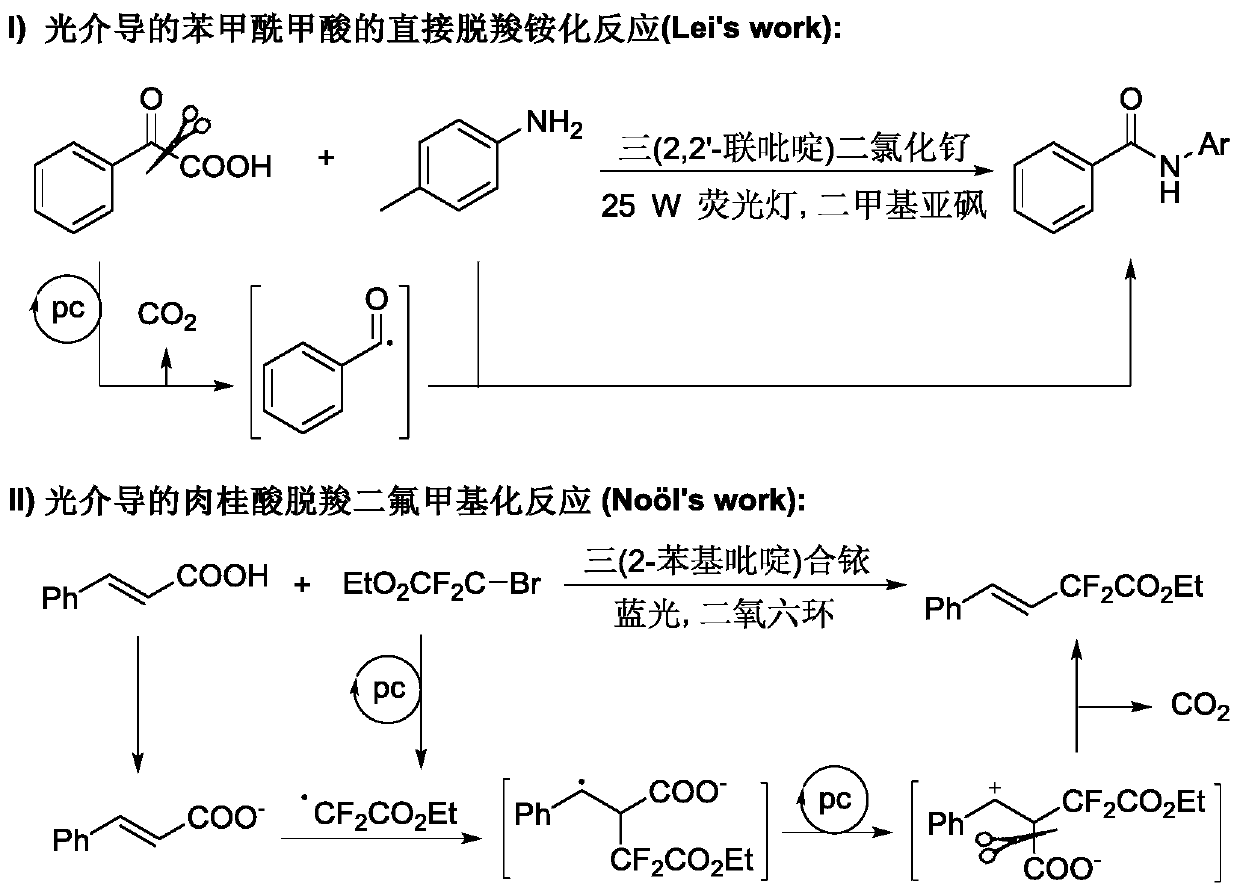
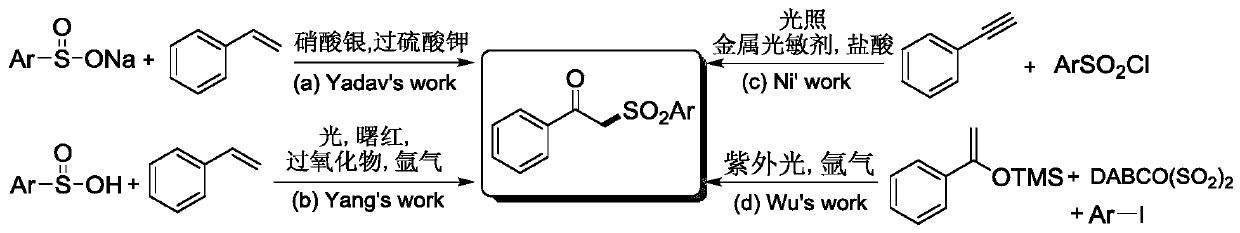
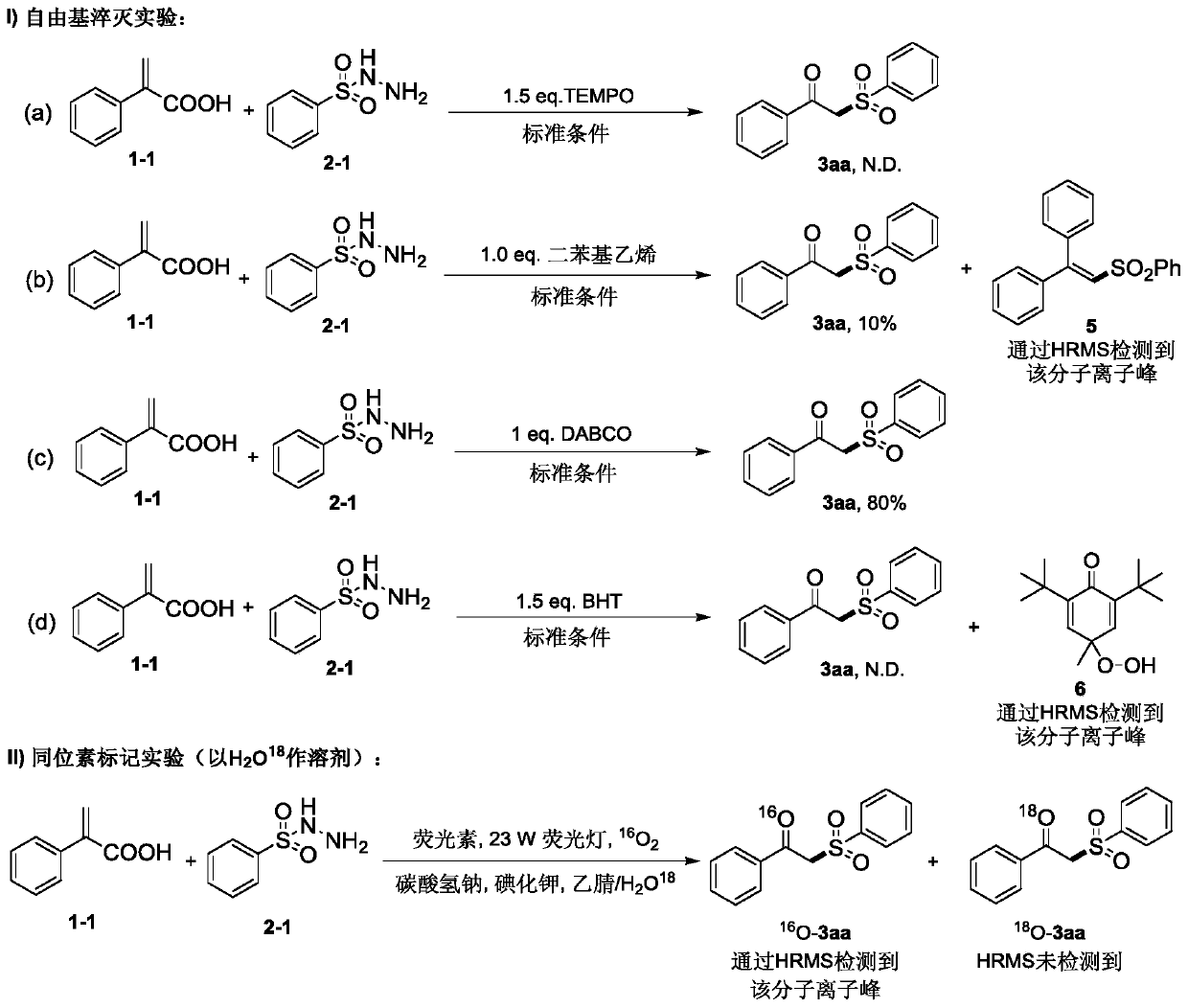
Method for preparing beta-ketosulfone compound through visible light mediated atopic acid decarboxylation ketonization reaction
A technology for decarboxylation ketones and compounds of atropic acid, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry and other directions, can solve problems such as excess, complicated deoxygenation operations, heavy metal residues, etc., and achieves mild reaction conditions and simple raw materials. Easy to obtain, no metal residue effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of compound 3aa (1-phenyl-2-(phenylsulfonyl)ethan-1-one), the preparation method is as follows:
[0052] (1) According to the molar ratio of 1:1.5:0.03:1:1, add atropic acid 1 (compound with structural formula 1-1), sulfonyl hydrazide 2 (compound with structural formula 2-1), fluorescein, alkali (NaHCO 3 ) and KI, fully dissolved in the mixed solvent of acetonitrile and water (the volume ratio of acetonitrile and water is 5.5:1), connected to the oxygen balloon under sealed conditions, and reacted under the illumination conditions of a compact fluorescent light of 23W, and the reaction process was performed by TLC Monitoring, detecting that the reaction of atropic acid 1 in the system is completed and the reaction can be ended;
[0053] (2) Concentrate the reaction mixture after the reaction under reduced pressure, add water to the concentrate, extract with ethyl acetate, combine the organic layers, wash with saturated brine and wash with anhydrous Na 2 SO...
Embodiment 2
[0057] Preparation of compound 3ab (1-phenyl-2-tosylethan-1-one), the preparation method is as follows:
[0058] (1) According to the molar ratio of 1:1.5:0.03:1:1, add atropic acid 1 (compound with structural formula 1-1), sulfonyl hydrazide 2 (compound with structural formula 2-2), fluorescein, alkali (NaHCO 3 ) and KI, fully dissolved in the mixed solvent of acetonitrile and water (the volume ratio of acetonitrile and water is 5.5:1), connected to the oxygen balloon under sealed conditions, and reacted under the illumination conditions of a compact fluorescent light of 23W, and the reaction process was performed by TLC Monitoring, detecting that the reaction of atropic acid 1 in the system is completed and the reaction can be ended;
[0059] (2) Remove acetonitrile by rotary evaporation after the reaction, add water to the obtained concentrated reaction mixture, extract with ethyl acetate, combine the organic phases, wash with saturated brine and wash with anhydrous Na 2 ...
Embodiment 3
[0063] Preparation of compound 3ac (2-((4-methoxyphenyl)sulfonyl)-1-phenylethan-1-one), the preparation method is as follows:
[0064] (1) According to the molar ratio of 1:1.5:0.03:1:1, add atropic acid 1 (compound with structural formula 1-1), sulfonyl hydrazide 2 (compound with structural formula 2-3), fluorescein, alkali (NaHCO 3 ) and KI, fully dissolved in the mixed solvent of acetonitrile and water (the volume ratio of acetonitrile and water is 5.5:1), connected to the oxygen balloon under sealed conditions, and reacted under the illumination conditions of a compact fluorescent light of 23W, and the reaction process was performed by TLC Monitoring, detecting that the reaction of atropic acid 1 in the system is completed and the reaction can be ended;
[0065] (2) After the reaction was completed, acetonitrile was removed by rotary evaporation, water was added to the obtained concentrated reaction mixture, extracted with ethyl acetate, the organic layers were combined, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com