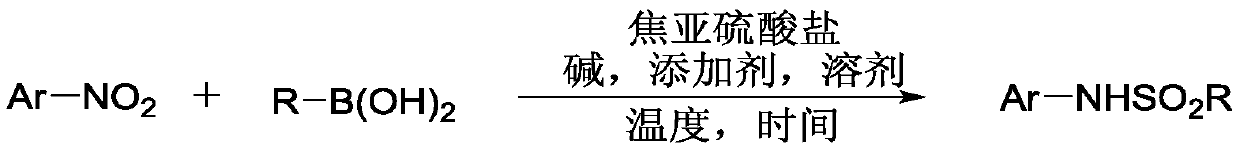
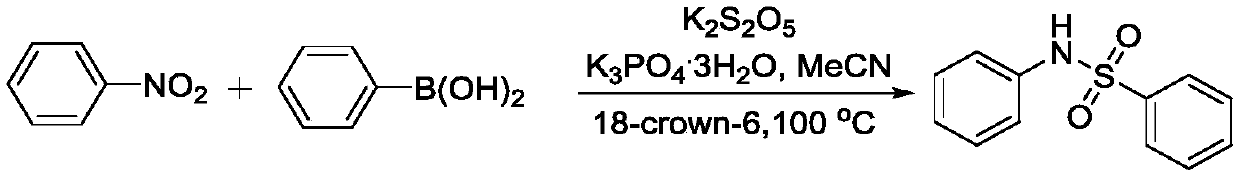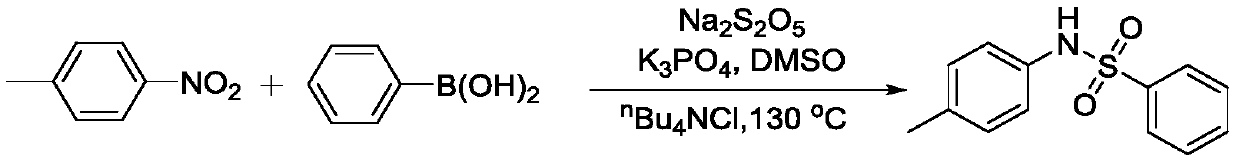Method for coupling nitroaromatic compound and boric acid compound to synthesize sulfonamide compound
A technology of boronic acid compounds and nitroaromatic hydrocarbons, applied in the preparation of sulfonic acid amides, the formation/introduction of sulfonyl/sulfinyl groups, organic chemistry, etc., can solve the lack of universality, sulfonyl chloride or sodium aryl sulfinate There are few types, difficult preparation and other problems, to achieve the effect of low price, wide substrate applicability and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] To the dry sealed tube, add 74 mg of the above nitroaromatics, 88 mg of phenylboronic acid, 267 mg of K 2 S 2 o 5 , 160mg of tripotassium phosphate trihydrate, 32mg of 18-crown-6 and 1.8mL of acetonitrile, then tighten the screw cap of the sealing tube, and react at 100°C for 12h. After the reaction was completed, it was filtered with diatomaceous earth, concentrated, and passed through a silica gel column to obtain 110 mg of the product with a yield of 79%.
[0035] Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0036] 1 H NMR (400MHz, CDCl 3 )δ7.83(d, J=7.3Hz, 2H), 7.53-7.50(m, 2H), 7.42(t, J=7.7Hz, 2H), 7.22(t, J=7.8Hz, 2H), 7.13- 7.07(m,3H); 13 C NMR (100MHz, CDCl 3 ) δ 138.9, 136.5, 133.1, 129.3, 129.1, 127.3, 125.4, 121.6.
Embodiment 2
[0038]
[0039] Add 82 mg of the above nitroaromatics, 146 mg of phenylboronic acid, 456 mg of Na 2 S 2 o 5 , 255mg of tripotassium phosphate, 334mg of tetrabutylammonium chloride and 3.6mL of dimethyl sulfoxide, and then tighten the screw cap of the sealing tube, and react at 130°C for 36h. After the reaction was completed, it was filtered with diatomaceous earth, concentrated, and passed through a silica gel column to obtain 89 mg of the product with a yield of 60%.
[0040] Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0041] 1 H NMR (400MHz, CDCl 3 )δ7.79(d, J=7.2Hz, 2H), 7.52(t, J=7.4Hz, 1H), 7.42(t, J=7.6Hz, 2H), 7.18(br, 1H), 7.03-6.96( m,4H),2.26(s,3H); 13 C NMR (100MHz, CDCl 3 ) δ 139.0, 135.5, 133.7, 132.9, 129.9, 129.0, 127.3, 122.4, 20.9.
Embodiment 3
[0043]
[0044] Add 92 mg of the above nitroaromatics, 146 mg of phenylboronic acid, 460 mg of K 2 S 2 o 5 , 166mg of potassium carbonate, 213mg of tetrabutylammonium bromide and 3mL of acetonitrile, then tighten the screw cap of the sealing tube, and react at 130°C for 24h. After the reaction was completed, it was filtered with diatomaceous earth, concentrated, and passed through a silica gel column to obtain 115 mg of the product with a yield of 73%.
[0045]Carry out nuclear magnetic resonance analysis to the product that present embodiment prepares:
[0046] 1 H NMR (400MHz, CDCl 3 )δ7.72(d, J=7.3Hz, 2H), 7.53(t, J=7.4Hz, 1H), 7.42(t, J=7.7Hz, 2H), 6.98(d, J=8.9Hz, 2H) ,6.82(br,1H),6.75(d,J=8.9Hz,2H),3.74(s,3H); 13 CNMR (100MHz, CDCl 3 ) δ 158.1, 138.9, 132.9, 129.0, 128.6, 127.3, 125.7, 114.5, 55.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com