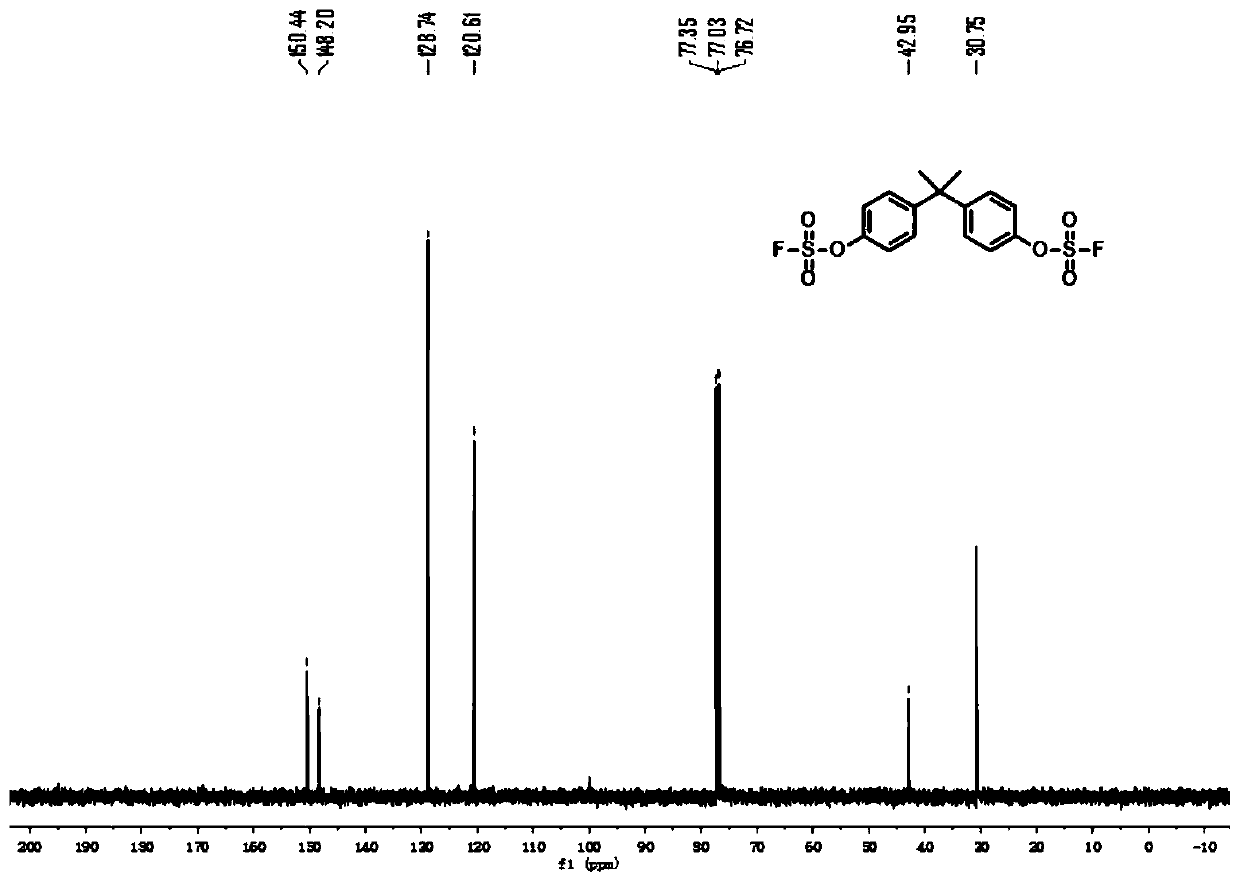
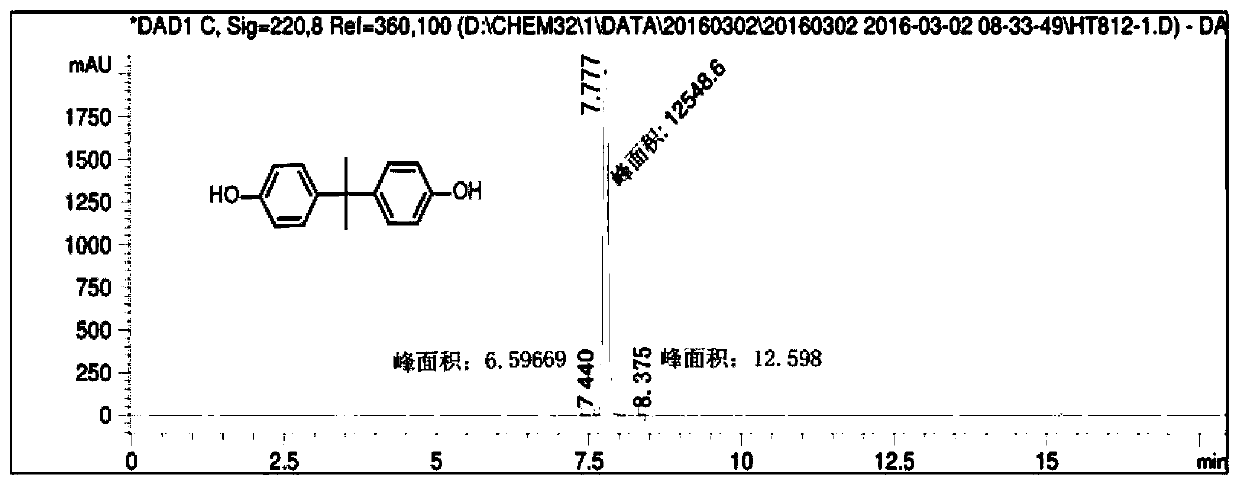
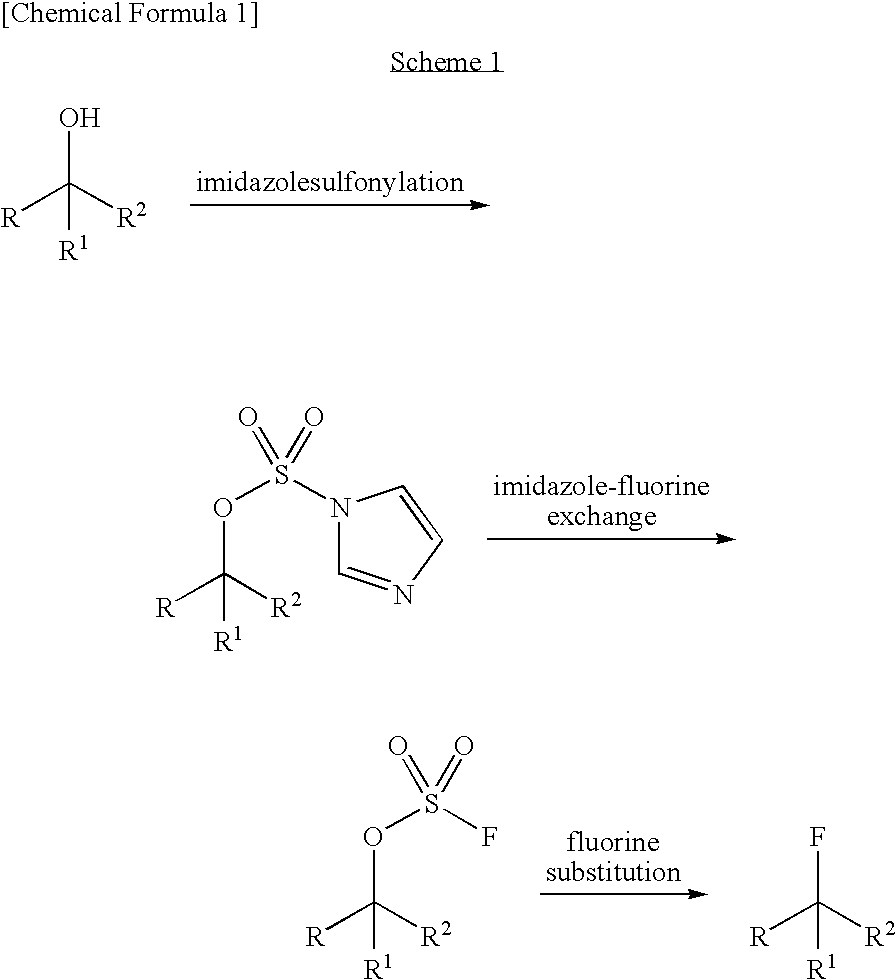
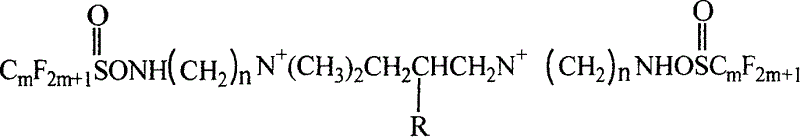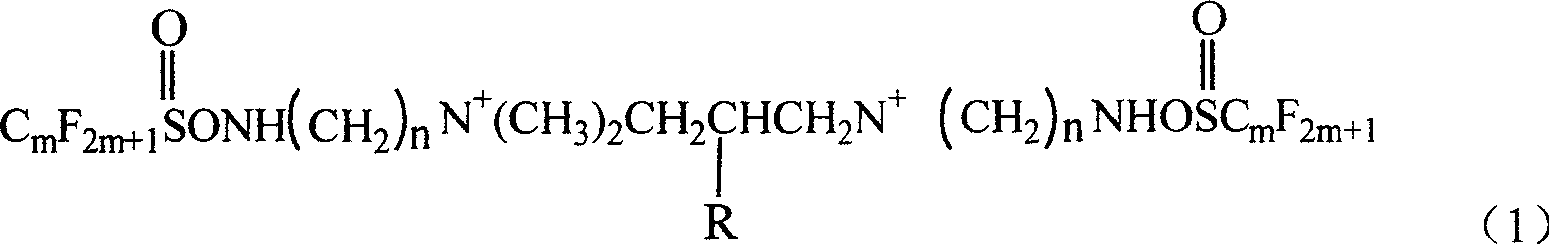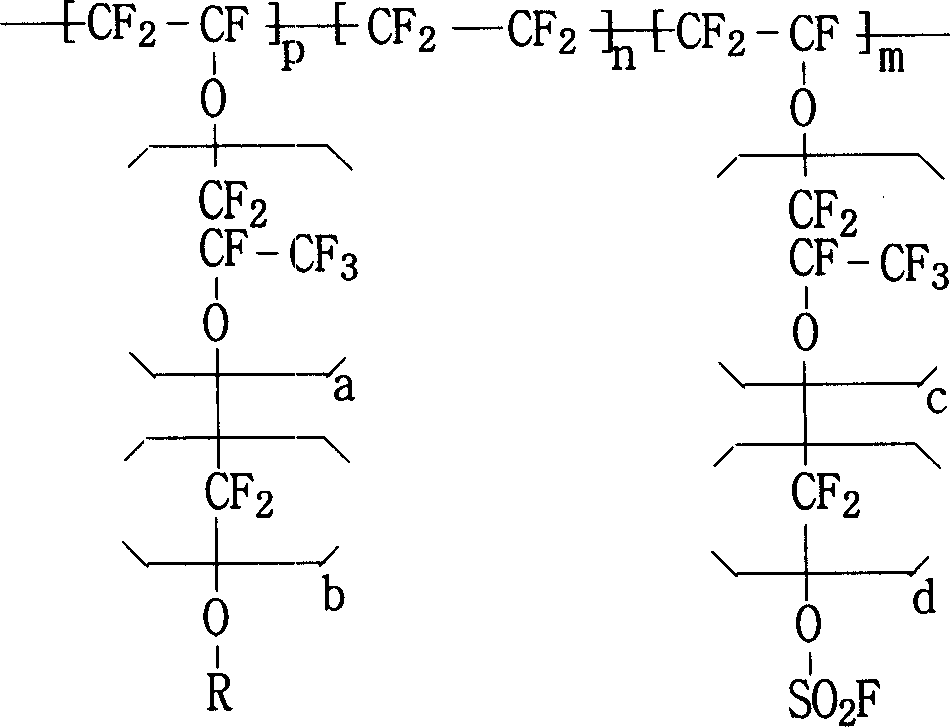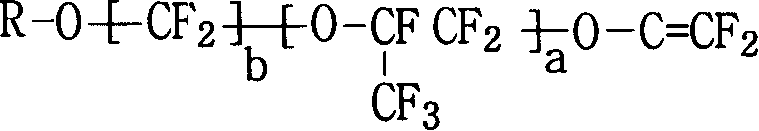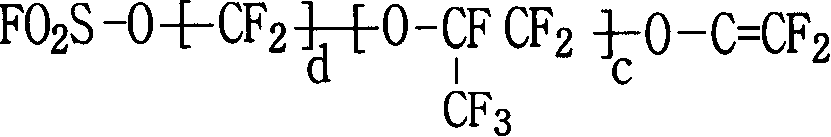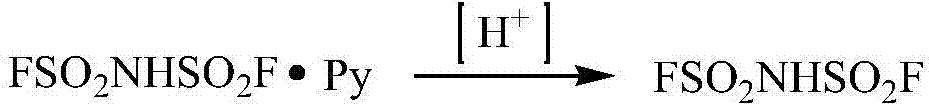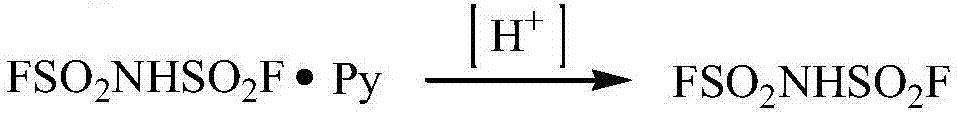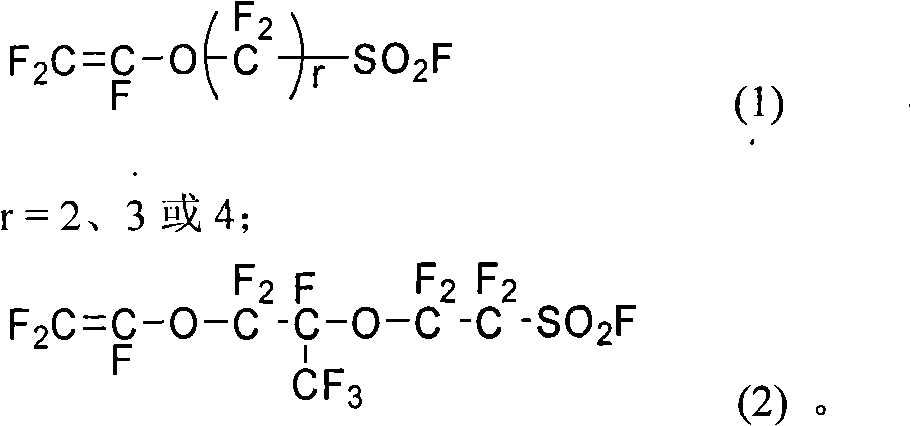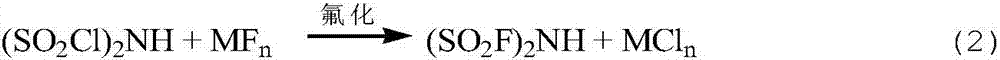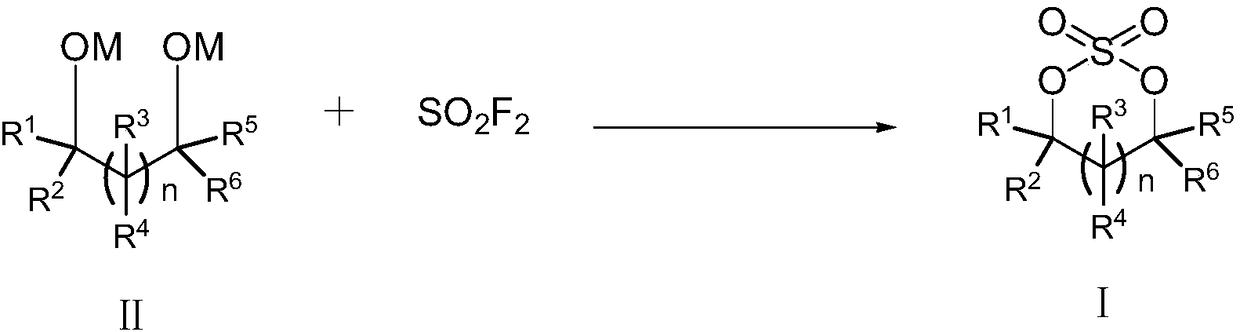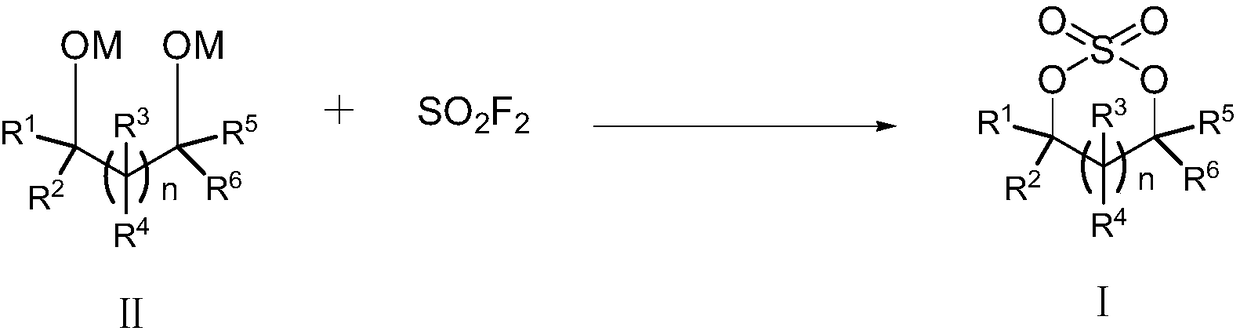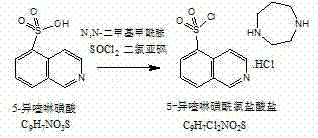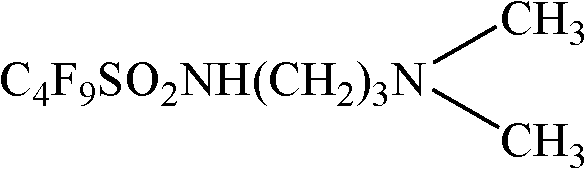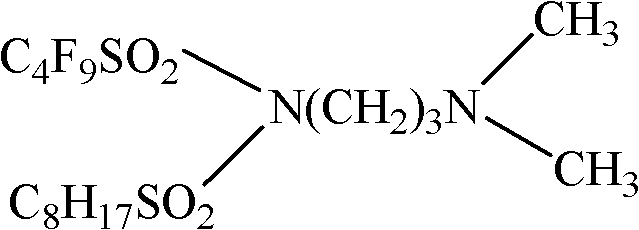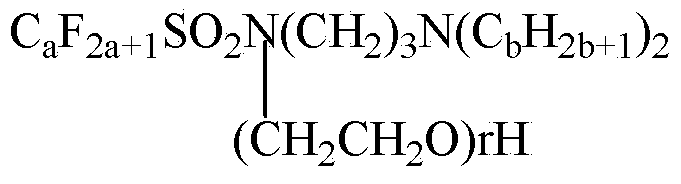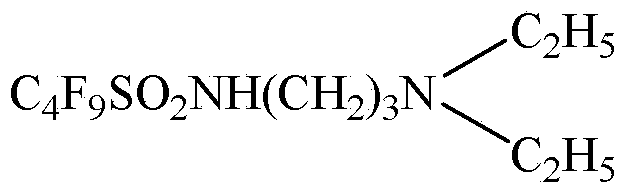Patents
Literature
242 results about "Sulfuryl fluoride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sulfuryl fluoride (also spelled sulphuryl fluoride) is an inorganic compound with the formula SO₂F₂. It is an easily condensed gas and has properties more similar to sulfur hexafluoride than sulfuryl chloride, being resistant to hydrolysis even up to 150 °C. It is neurotoxic and a potent greenhouse gas, but is widely used as a fumigant insecticide to control termites.
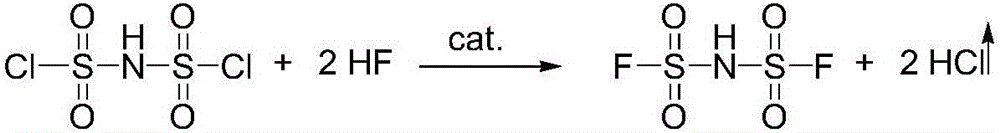
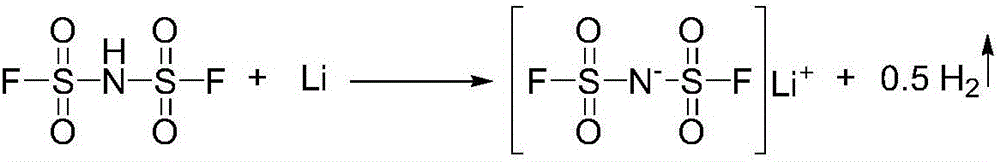
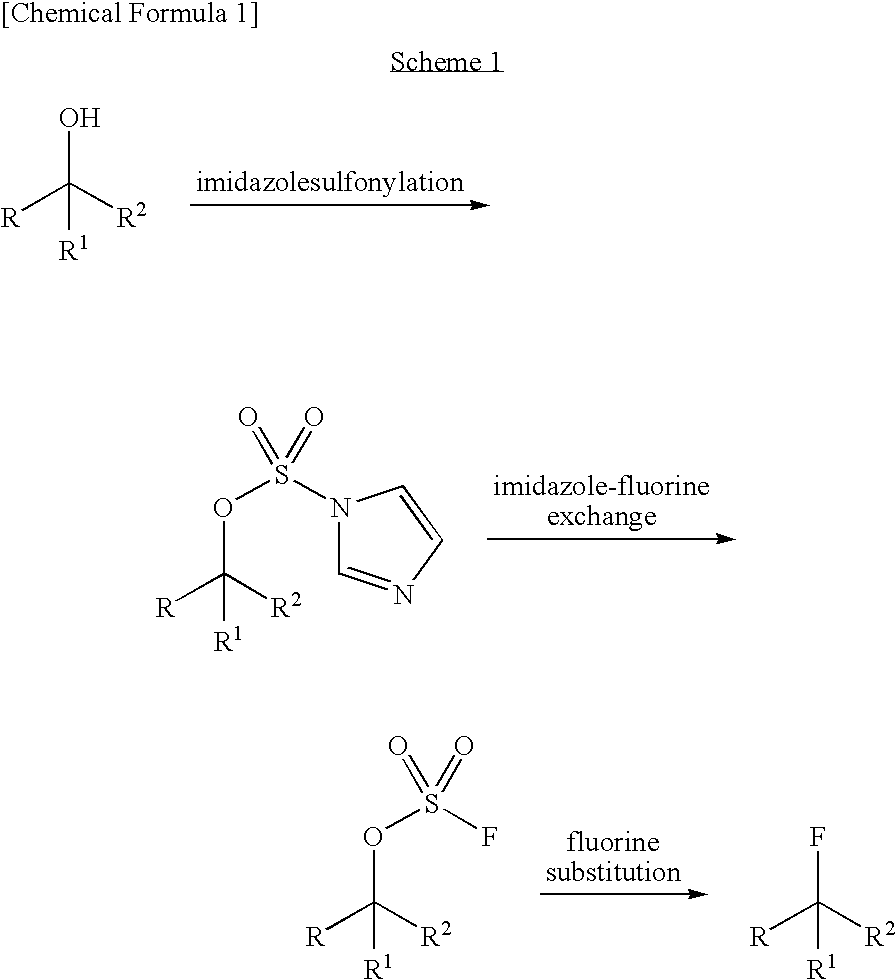
Preparation method of imidodisulfuryl fluoride lithium salt
The invention relates to the field of chemical synthesizing, in particular to a preparation method of imidodisulfuryl fluoride lithium salt. The preparation method includes the steps of firstly, allowing chlorosulfonic acid and chlorosulfonyl isocyanate to react in the presence of catalyst to obtain dichlorosulfimide; secondly, allowing dichlorosulfimide and hydrogen fluoride to react in the presence of catalyst to obtain imidodisulfuryl fluoride; thirdly, allowing imidodisulfuryl fluoride to react with compound containing lithium to obtain the imidodisulfuryl fluoride lithium salt. The preparation method has the advantages that the chlorosulfonic acid and chlorosulfonyl isocyanate are used as the raw materials in the first-step reaction, the generation of waste gases such as SO2 and HCl is avoided, and environment protection requirements are satisfied.
Owner:SHANGHAI CHEMSPEC CORP +1
SiO2/perfluorinated sulfonic resin compound proton exchange membrane and preparation method thereof
ActiveCN101773793AHigh strengthImprove conductivitySemi-permeable membranesCell electrodesHydrogenCerium
The invention relates to a SiO2 / perfluorinated sulfonic resin compound proton exchange membrane and a preparation method thereof, which is composed of perfluorinated sulfonic resin and SiO2 doped with cerium or / and manganese. The perfluorinated sulfonic resin is H-type resin with sulfonic acid groups or F type resin with sulfuryl fluoride groups with the equivalent value of 600-1,300g / mmol. The compound proton exchange membrane prepared in the invention has higher proton conductivity and mechanical strength, can effectively prevent penetration of hydrogen and methanol, and is favor of improvement of fuel cell property.
Owner:SHANDONG DONGYUE WEILAI HYDROGEN ENERGY MATERIAL CO LTD
Fluorine-carbon sufactant and preparing method
InactiveCN1743062AReduce surface tensionImprove solubilityTransportation and packagingMixingEthylenediamineDichloropropane
The present invention discloses a new-type double-ion type fluorocarbon surfactant. It is a double-ion type fluorocarbon surfactant using hydrophilic monoester dichloropropane as new-type hydrophilic flexible space group of coupling group, its chemical name is N,N,N',N'-tetramethyl-N,N'-bis [(perfluoroalkyl sulamine)-alkyl]-2-monoester group-propylenediammonium. Said surfactant utilizes reaction of perfluoroalkyl sulfuryl fluoride and N,N-dimethyl-1,2-ethylenediamine or N,N-dimethyl-1,3-propylenediamine to obtain intermediate product N-[(dimethylamino)-alkyl] perfluoroalkyl sulfamine, then makes the intermediate product and monoester dichloropropane undergo the process of quaterisation so as to obtain the invented product.
Owner:NANJING TECH UNIV
Fluor resin with sulfuryl fluoride and aether terminal group lateral group, synthesizing method and application thereof
The invention pertains to the field of fluoro-containing macromolecular materials, which provides a per-fluorocarbon resin with sulfonic acid fluoride and lateral group of aether end group and the per-fluorocarbon resin is the per-fluorocarbon resin obtained by ternary copolymerization of alkyl ether end group vinyl ether monomer (A), tetrafluoroethylene (B) and vikane ether end group monomer (C). The mol content percentage of the three monomers occupying in the polymer is that: A is equal to 0.01-5 percent; B is equal to 48-84.9 percent; C is equal to 15-47 percent; a per-fluorocarbon ion exchange membrane prepared not only has various chemical mediator resistance, but also has high electrical conductivity, high mechanical strength and low membrane resistance and is suitable for being used in fuel batteries or chlor-alkali electrolytic bath. The invention further provides a preparation method and application of the resin.
Owner:SHANDONG HUAXIA SHENZHOU NEW MATERIAL
Preparation method for lithium bis(fluorosulfonyl)imide
The invention discloses a preparation method for lithium bis(fluorosulfonyl)imide. The preparation method comprises the following steps: 1) preparing fluorine sulfamide lithium; 2) preparing sulfuryl fluoride, and pumping the sulfuryl fluoride into the fluorine sulfamide lithium solution, and adding an acid-binding agent to prepare bifluorine sulfimide ammonium salt; 3) dissolving the bifluorine sulfimide ammonium salt in water solution, to obtain bifluorine sulfimide water solution through the acid resin exchange; 4) adding lithium carbonate, regulating the PH value to be neutral, filtering and removing undissolved substances, depressurizing and removing the most of the water, and adding a weak-polarity organic solvent to separate out a coarse product of the lithium bis(fluorosulfonyl)imide, further depressurizing and drying; and 5) adding a polarity solvent to the coarse product of the lithium bis(fluorosulfonyl)imide, stirring and dissolving, filtering and removing the undissolved substances, depressurizing and removing a strong-polarity solvent, and adding the weak-polarity organic solvent to perform the recrystallization, and depressurizing and drying to obtain a product after filtering. The preparation method is simple, the reaction time is short, the yield is high, and metal ion and negative ion impurities can be effectively controlled, so that the product with the high purity can be obtained.
Owner:常德市大度新材料有限公司
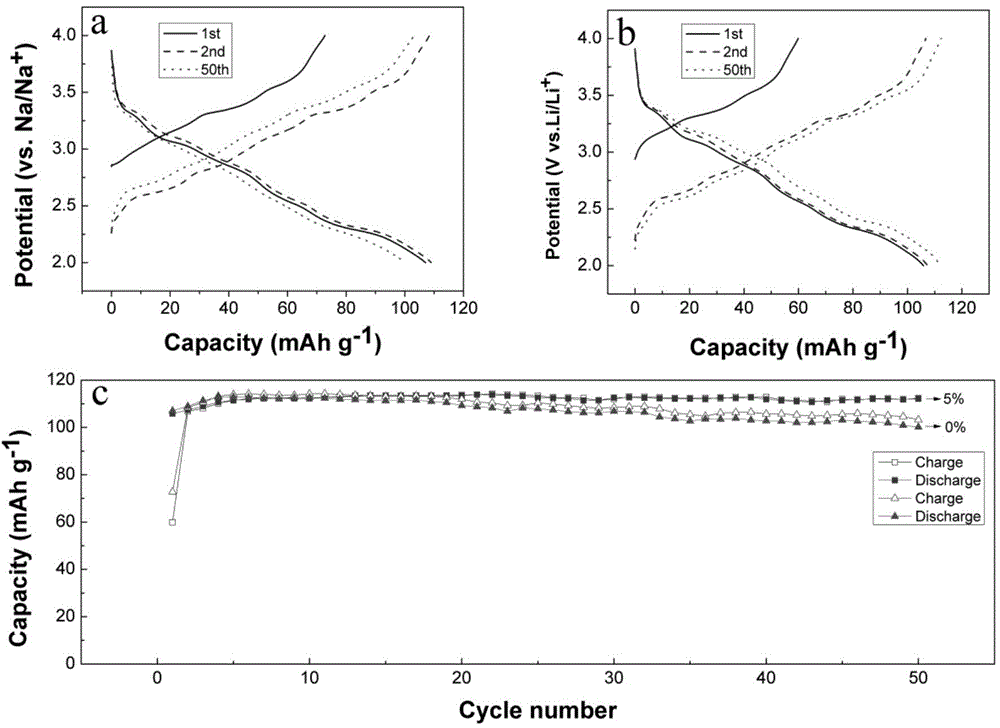
Flame retardant sodium-ion battery electrolytic solution and application thereof
ActiveCN104900879AImprove flame retardant performanceLittle impact on electrochemical performanceCell electrodesSecondary cellsOrganic solventCarbonate ester
The invention relates to a flame retardant sodium-ion battery electrolytic solution and application thereof. The electrolytic solution comprises the following ingredients with the concentration: 0.001-2mol / L sodium salt, carbonate esters and / or ether organic solvents, 0.1-50% of a flame retardant additive and 0-0.5mol / L other functional additives. The flame retardant sodium-ion battery electrolytic solution is applied into a sodium primary battery, a sodium secondary battery and a sodium ion battery. A sulfuryl fluoride base group-containing compound has good flame retardance, has little influence on the electrochemical performance and can be used as a novel electrolytic solution flame retardant additive; the flame retardant additive is added into the electrolytic solution, so that the combustibility of the electrolytic solution can be reduced. A phosphonitrile base group-containing compound has good flame retardance, has little influence on the electrochemical performance, and can be used as a novel electrolytic solution flame retardant additive; the flame retardant additive is added into the electrolytic solution, so that the combustibility of the electrolytic solution can be reduced. After the electrolytic solution is applied to the sodium battery, the combustion possibility of the electrolytic solution is zero.
Owner:SHANDONG ZESHI NEW MATERIALS TECH CO LTD
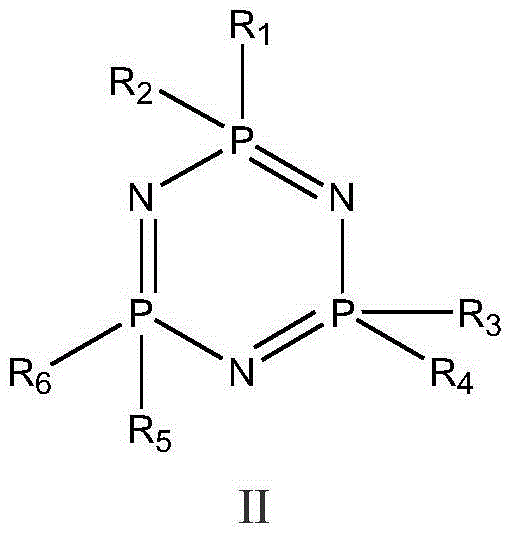
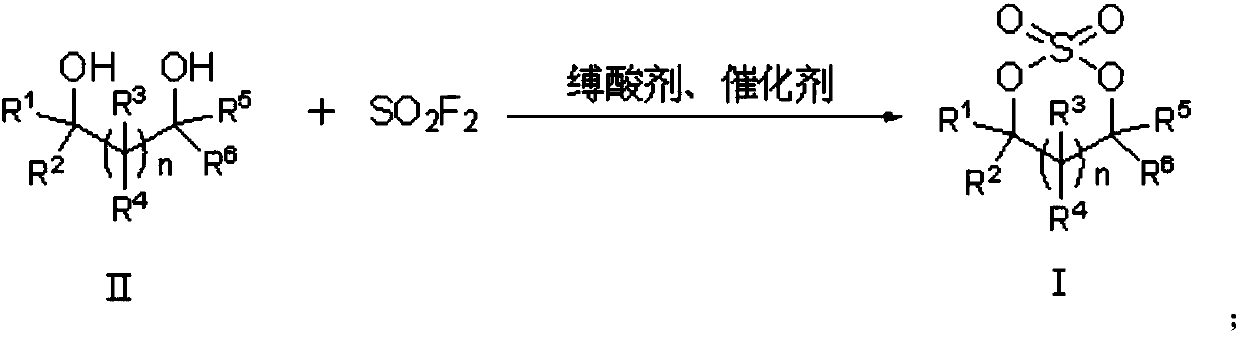
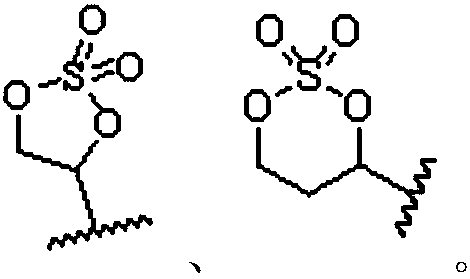
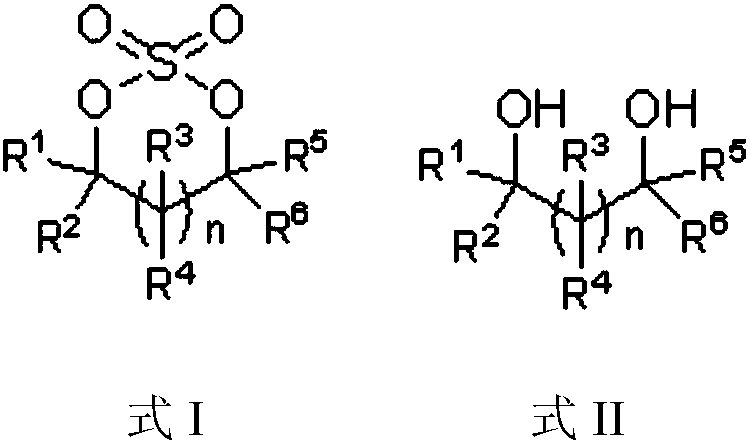
Preparation method of cyclic sulfate
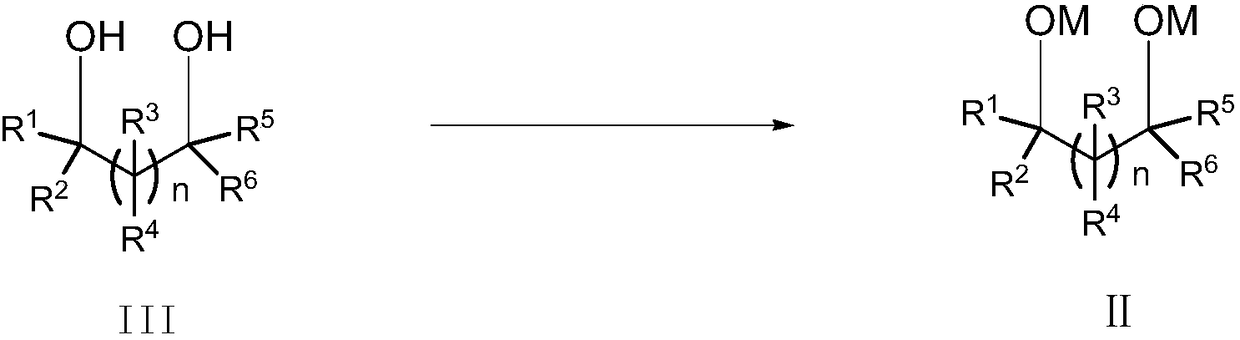
The invention relates to the field of organic synthesis, in particular to a preparation method of cyclic sulfate. The structural formula of cyclic sulfate is shown as formula I. The preparation methodof cyclic sulfate comprises the following step: cyclic sulfate in the formula I is prepared from a compound in formula II and sulfuryl fluoride by reacting in the presence of an acid binding agent and a catalyst. The preparation method of cyclic sulfate adopts short reaction step, cyclic sulfate can be prepared with the one-step reaction, cost of raw and auxiliary materials is low, and expensiveraw materials and oxidizing agent are not used.
Owner:SHANGHAI CHEMSPEC CORP +1
Polymerization method of (ammonium) sulfate connexon polymer
The invention relates to a polymerization method of an (ammonium) sulfate connexon polymer. The polymerization method comprises a step of subjecting a hydroxyl-containing monomer or an amino-containing monomer and a sulfuryl fluoride monomer to condensation polymerization on through a one-pot process under alkaline conditions. Compared with a traditional polyester synthesis method, the method is cost-saving; reaction conditions are mild and easy to control; reaction flow is simple, and operation is easy; and a post-treatment process is simple, environmental pollution is small, and industrial production is facilitated. In addition, a bisphenol type polysulfate compound synthesized in the invention has excellent mechanical properties, dielectric properties, tolerance and wear resistance.
Owner:内蒙古图微新材料科技有限公司
Preparation method of lithium bis (fluorosulfonyl) imide
PendingCN111620315AReduce usageThe reaction process is mild and easy to controlNitrosyl chlorideImideLithium oxide
The invention discloses a preparation method of lithium bis (fluorosulfonyl) imide. The preparation method comprises the following steps: (1) in the presence of an ammonia source, an organic solvent,fluoride salt and an initial amount of sulfuryl fluoride, slowly introducing organic alkali while continuously introducing the balance of sulfuryl fluoride until the reaction is finished, and directlycarrying out reduced pressure distillation on the reaction solution to obtain an intermediate, namely the difluorosulfonyl imide salt; and (2) in the presence of an organic solvent, adding lithium oxide powder into the intermediate imidodisulfuryl fluoride salt, filtering, concentrating, adding a non-aqueous poor solvent, and crystallizing to obtain lithium bis (fluorosulfonyl) imide.
Owner:SHANGHAI HUAYI GRP CO +1
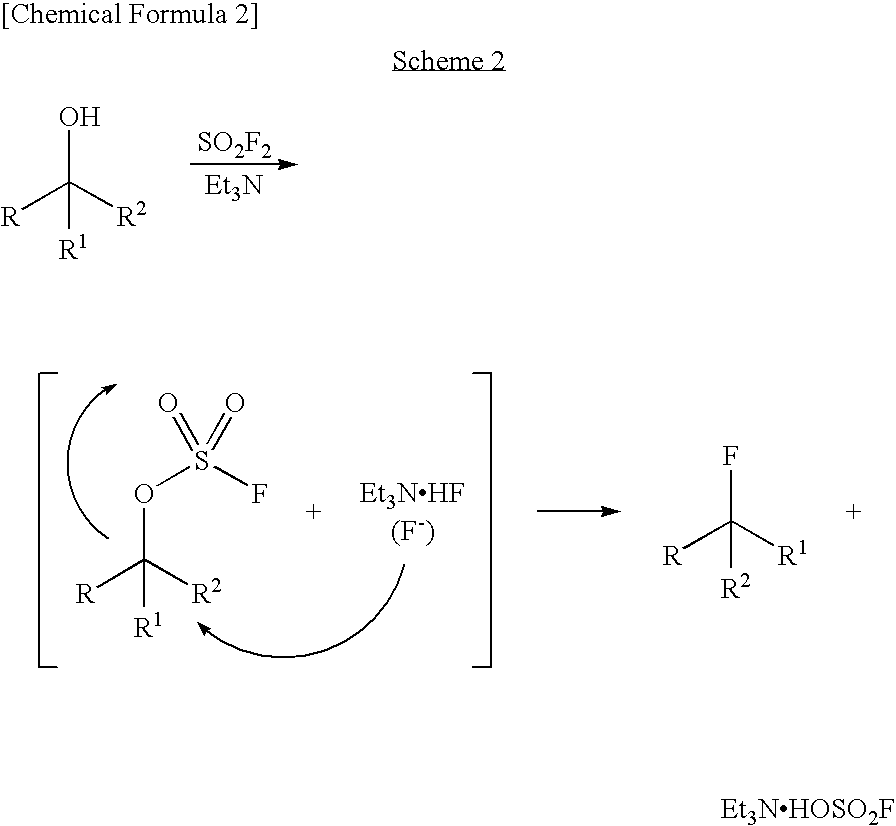
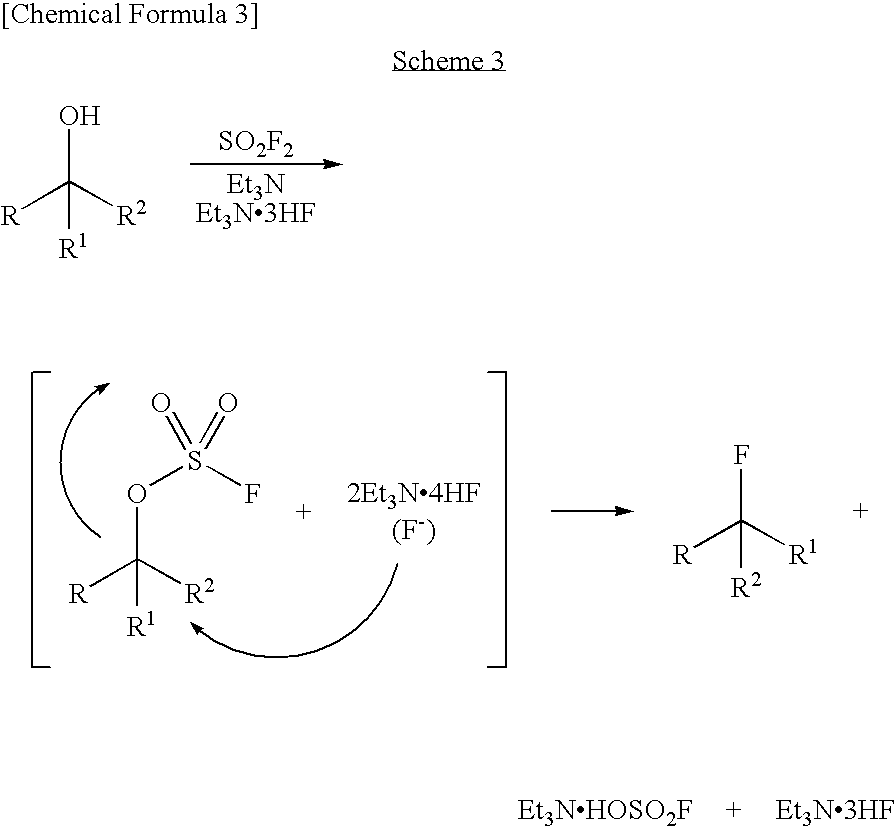
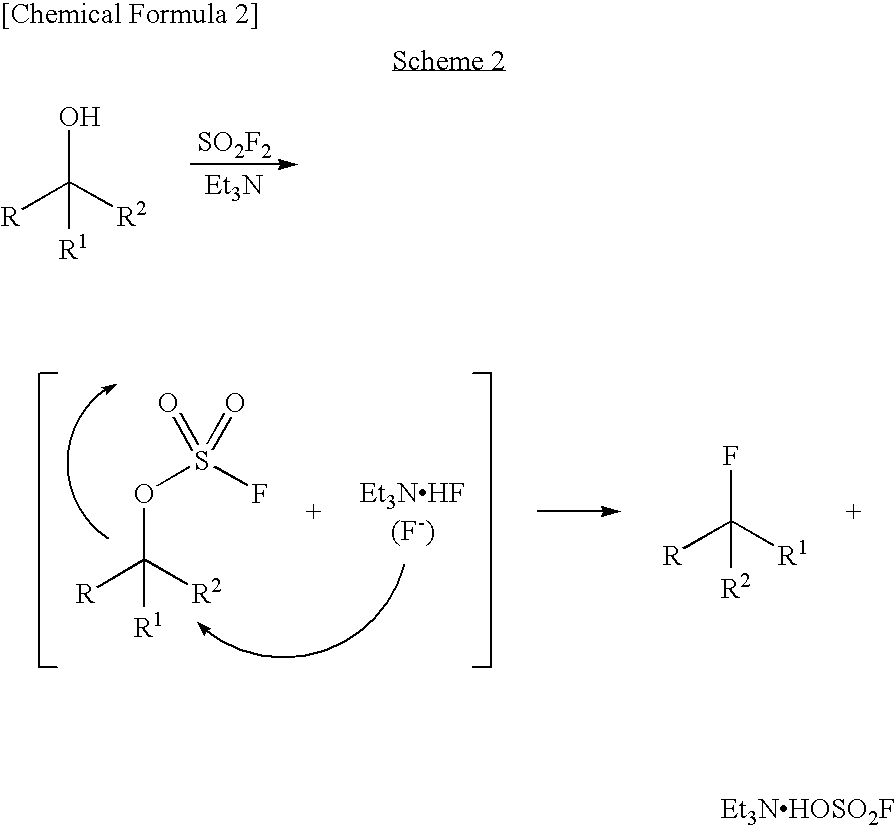
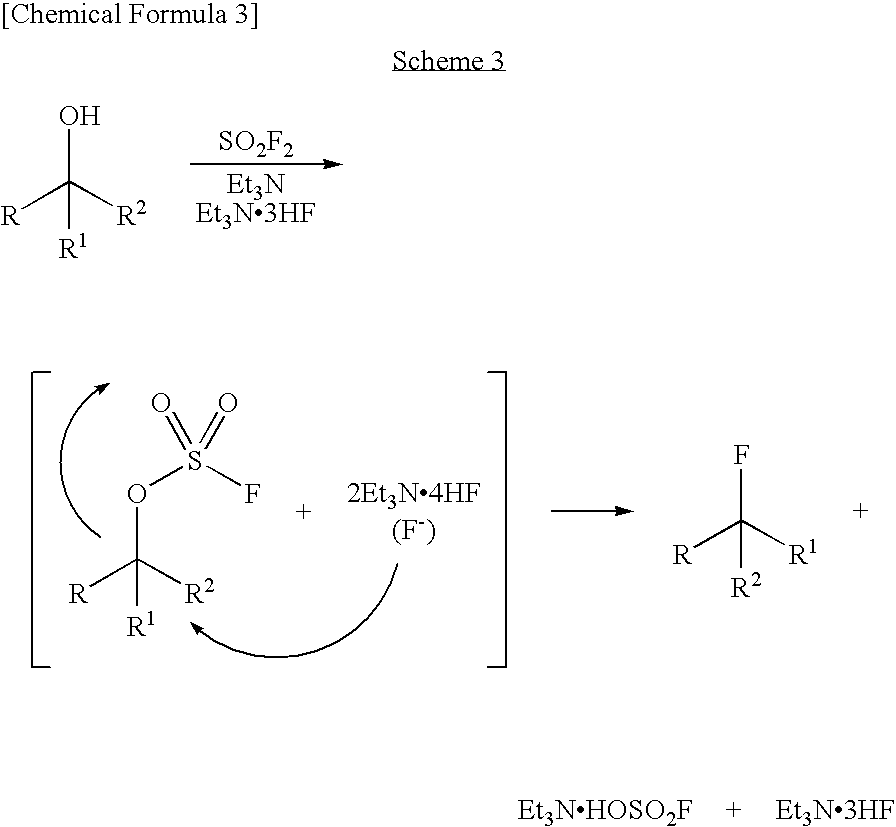
Process for Production of Fluoro Derivative
InactiveUS20080125589A1High selectivityEfficient productionSugar derivativesOrganic compound preparationHydrogen fluorideOrganic base
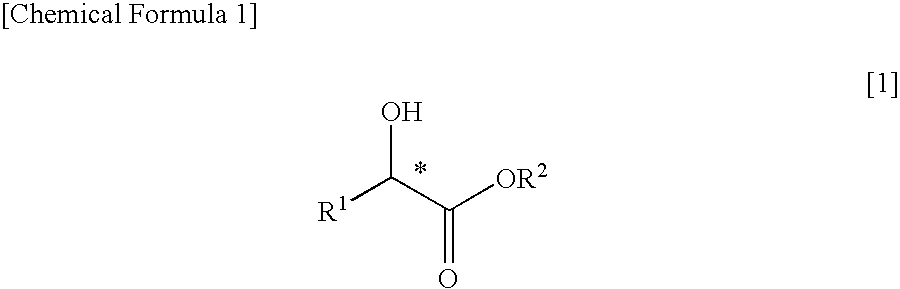
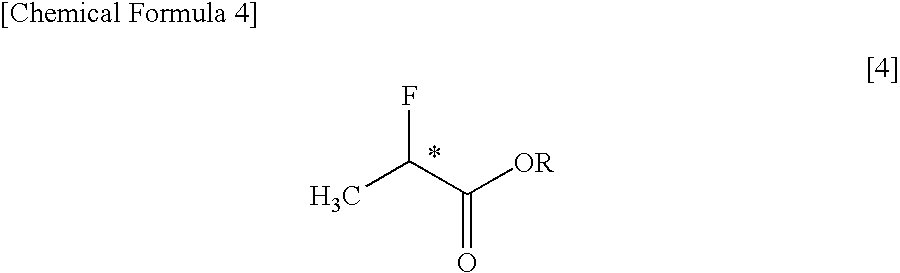
It was found that a fluoro derivative can be produced by reacting a hydroxy derivative with sulfuryl fluoride (SO2F2) in the presence of an organic base or in the presence of an organic base and “a salt or complex comprising an organic base and hydrogen fluoride”. According to the present production process, it is not necessary to use perfluoroalkanesulfonyl fluoride, which is not preferable in industrial use, and it is possible to advantageously produce optically-active fluoro derivatives, which are important intermediates of medicines, agricultural chemicals and optical materials, specifically 4-fluoroproline derivatives, 2′-deoxy-2′-fluorouridine derivatives, optically-active α-fluorocarboxylate derivatives, and the like, even in a large scale.
Owner:CENT GLASS CO LTD
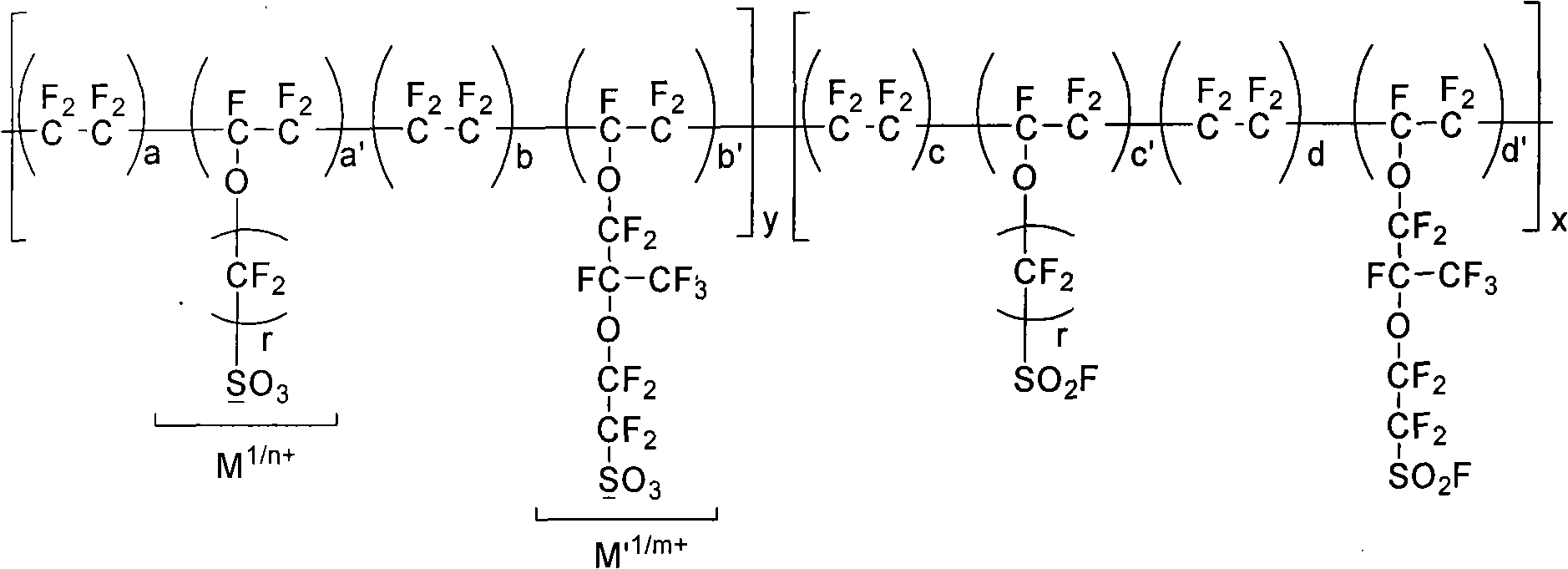
Functional perfluoro resin and preparation method thereof
ActiveCN101775095AEvenly distributedImprove antioxidant capacityOrganic diaphragmsFinal product manufactureVinyl etherTetrafluoroethylene
The invention provides a functional perfluoro resin with sulfuryl fluoride and sulphonate lateral-group, belonging to the field of fluorine-containing high molecular materials. The functional perfluoro resin is prepared by multiply copolymerizing tetrafluoroethylene, two sulfuryl fluoride lateral-group vinyl ether monomers of different structures, two sulphonate lateral-group vinyl ether monomers which are respectively correspond to the sulfuryl fluoride lateral-group vinyl ether monomers of different structures, wherein the total mole fraction of the tetrafluoroethylene is 50-90.9 percent of a copolymer, the total mole fraction of the sulfuryl fluoride lateral-group vinyl ether monomers is 8.8-49.99 percent, and the total mole fraction of the sulphonate lateral-group vinyl ether monomers is 0.01-0.3 percent. A perfluoro ion exchange membrane prepared by the functional perfluoro resin not only has chemical mediator resistance, but also has high conductivity, mechanical strength and dimension stability, low membrane resistance and long service life, and is suitable for fuel cells or chlor-alkali electrolytic cells. The invention also provides a preparation method and application of the functional perfluoro resin.
Owner:SHANDONG DONGYUE WEILAI HYDROGEN ENERGY MATERIAL CO LTD
Perfluorosulfonic composite proton exchange membrane for fuel cell
InactiveCN101777659AHigh mechanical strengthImprove proton conductivityCell component detailsFuel cell detailsManganeseCerium
The invention provides a perfluorosulfonic composite proton exchange membrane for a fuel cell and a preparation method thereof. Perfluorosulfonic resin formed by perfluorosulfonic resin, M-type perfluorosulfonic resin and porous polymer enhanced materials is H-type resin with sulfonic acid groups (-SO3-) or F-type resin with sulfuryl fluoride groups (-SO2F-). The M-type perfluorosulfonic resin is cerium or / and manganese metal ion type perfluorosulfonic resin formed in a way that cerium or / and manganese ions are fully exchanged with sulfonic acid groups or sulfuryl fluoride groups in perfluorosulfonic resin. Cerium or / and manganese ions can be evenly distributed on the membrane body, the thermal stability is high, the heat treatment temperature of the composite membrane is improved, the combination of the porous polymer enhanced materials with the perfluorosulfonic resin is facilitated, the prepared composite proton exchange membrane has good mechanical strength and proton conductivity capacity, and the performance of the fuel cell is improved.
Owner:SHANDONG HUAXIA SHENZHOU NEW MATERIAL
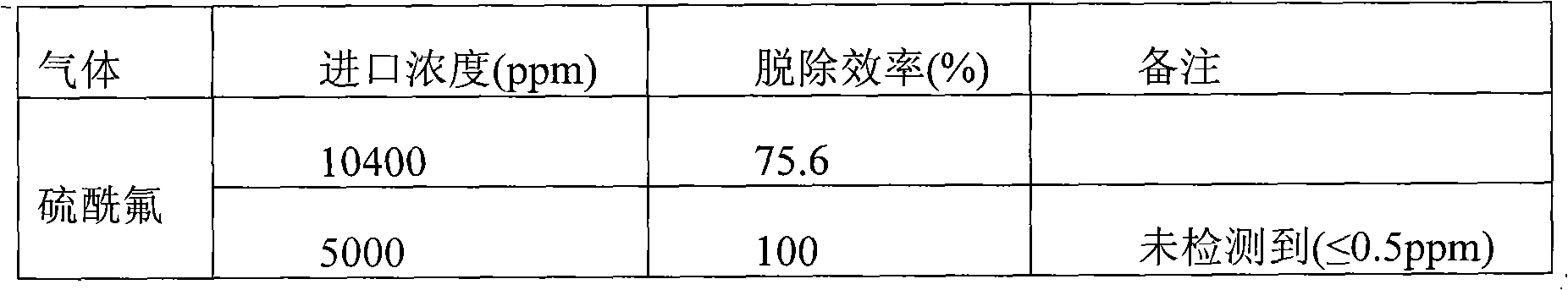
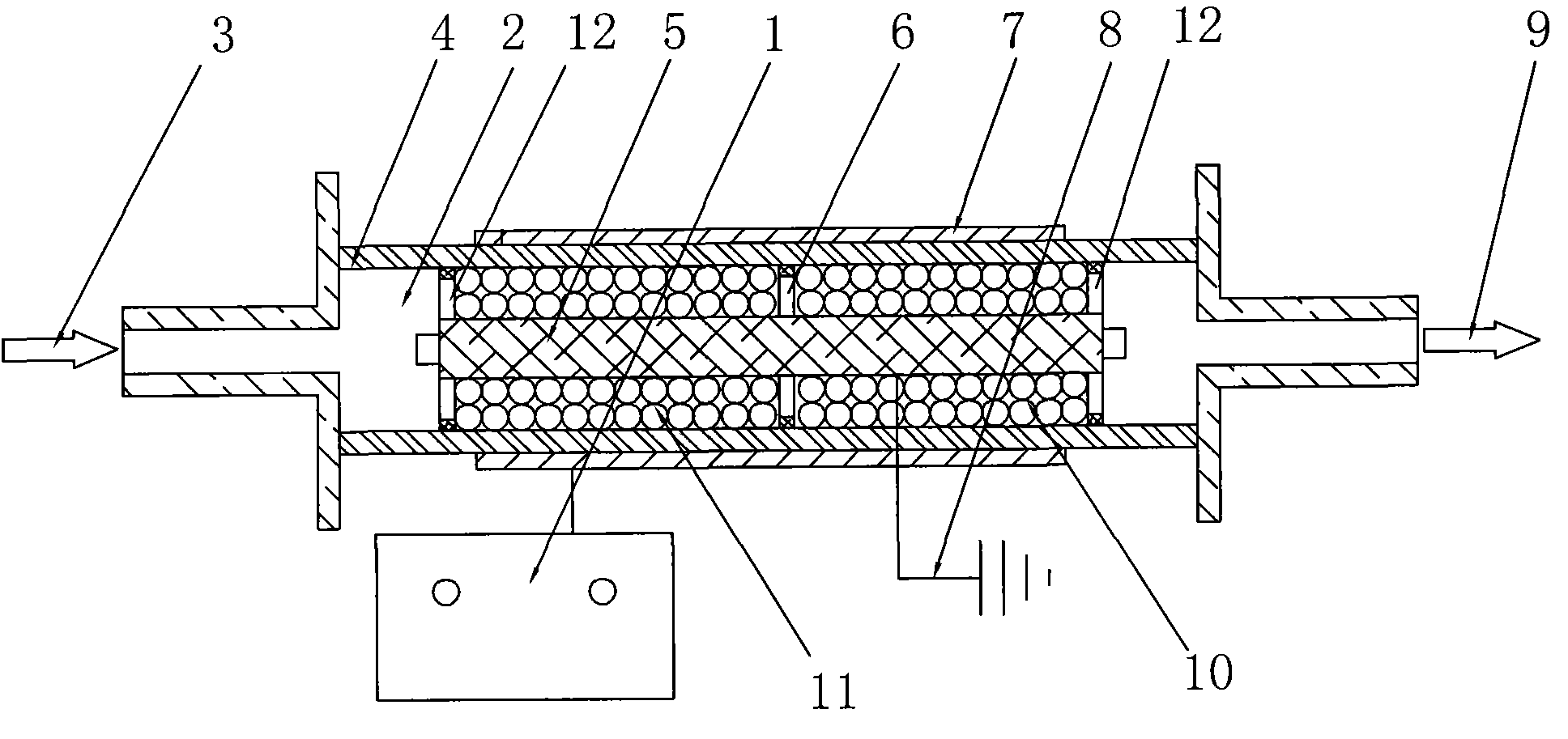
Innocent treatment method for sulfuryl fluoride gas
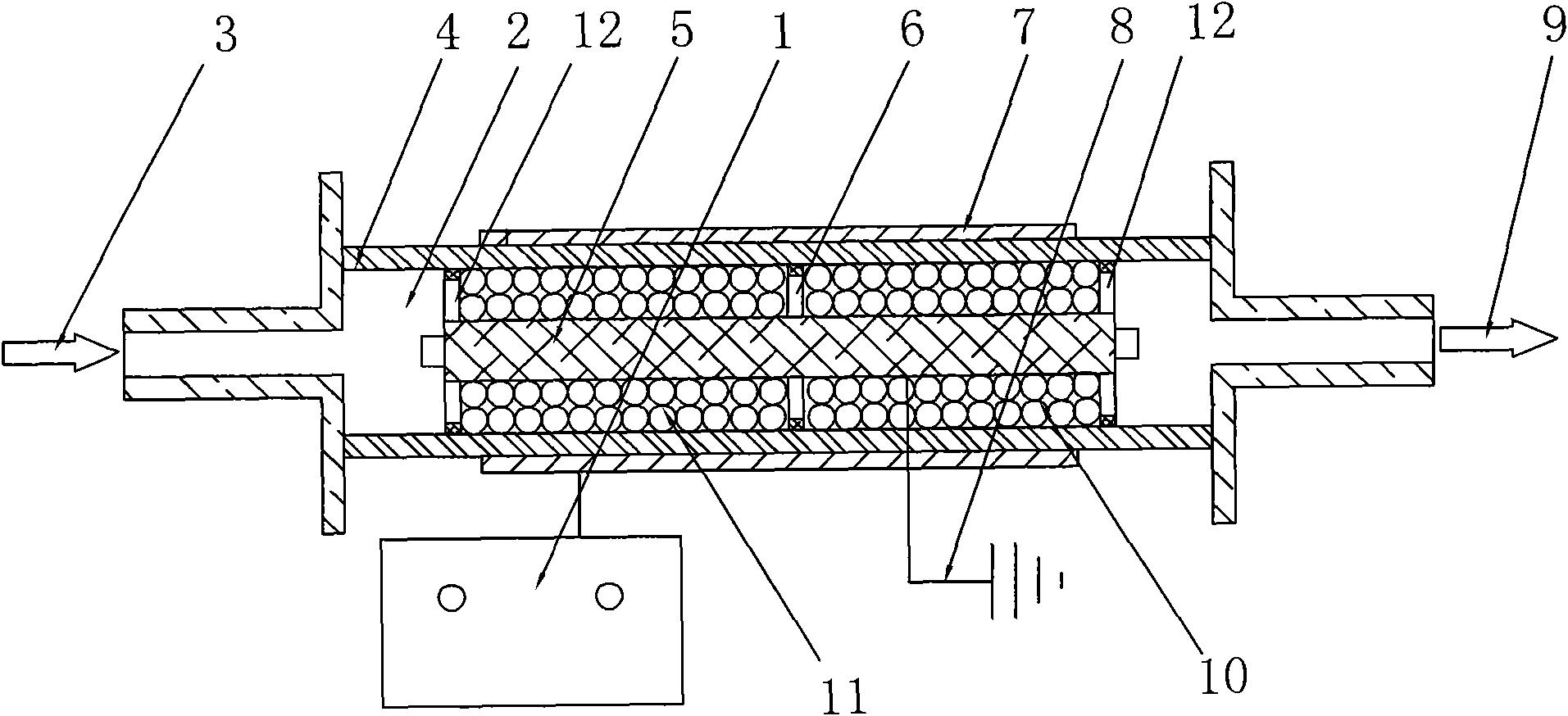
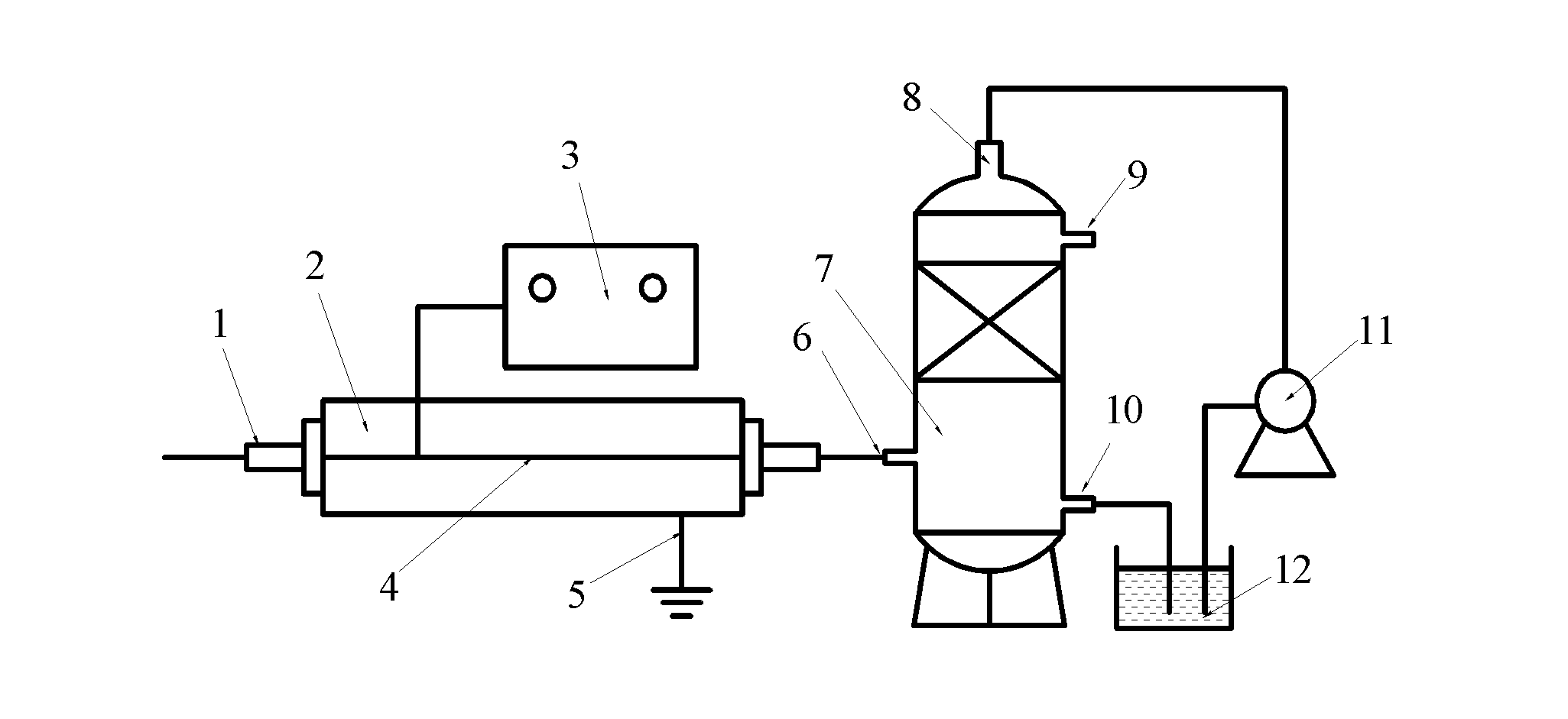
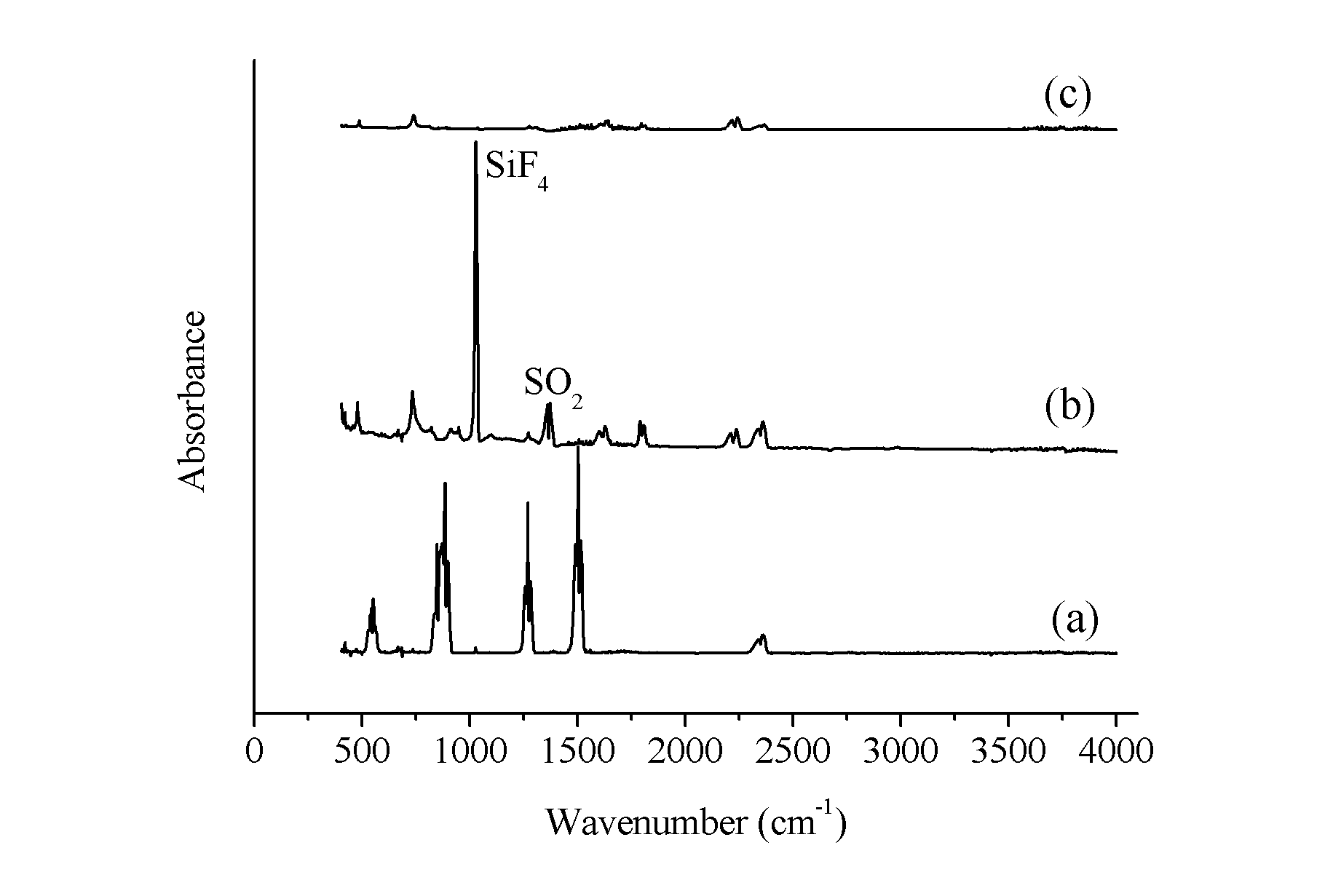
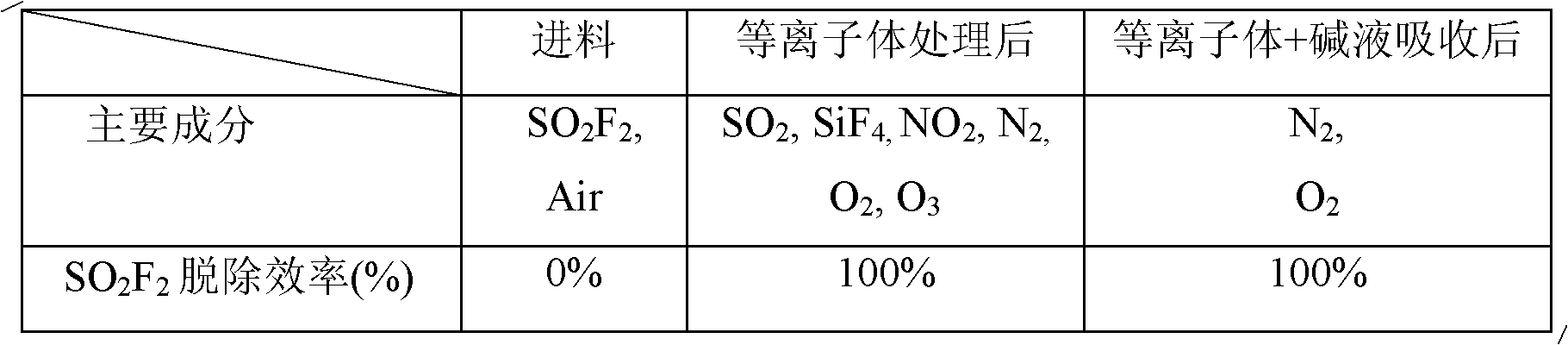
ActiveCN101972587AImprove removal efficiencyImprove utilization efficiencyDispersed particle separationElectric dischargeHigh energy
The invention belongs to the technical field of gas pollutant treatment, in particular relates to an innocent treatment method for sulfuryl fluoride gas. The method comprises the following steps of: dividing a plasma reactor serving as a reactor into two stuffing cavities between two electrodes by gas distribution baffles at both ends and a porous baffle in the middle; and introducing sulfuryl fluorid-containing gas into the plasma reactor from a gas inlet, performing oxidization reaction and degradation reaction on the sulfuryl fluorid-containing gas and high-energy electrons and active particles which are generated by electric discharge and reacting with secondary stuffing in the plasma reactor and absorbing under the action of nonequilibrium plasma to generate non-toxic and innocent gas without greenhouse effect and discharging from a gas outlet. The method has the advantages that the sulfuryl fluoride is oxidized and degraded under the action of the nonequilibrium plasma by adopting a mode that the discharge plasma and the stuffing are in the same electric field simultaneously, and reacts with the stuffing loaded in the reactor simultaneously to be absorbed so as to promote the reaction to perform towards the innocent direction, and improve the desorption efficiency, and thus the utilization ratio of energy is improved.
Owner:ZHEJIANG UNIV OF TECH +1
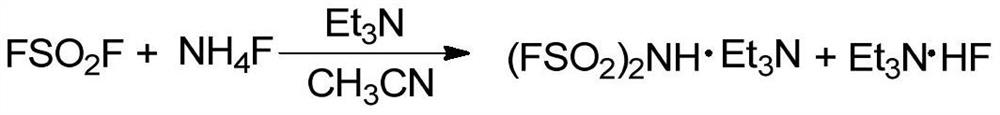
Preparation method of bis(fluorosulfonyl)amine
ActiveCN107986248AStrong continuous operationSimple processNitrosyl chlorideDistillationStrong acids
The invention discloses a preparation method of bis(fluorosulfonyl)amine. The preparation method comprises the following steps of enabling SO2F2 (sulfuryl fluoride), NH4F (ammonium fluoride) and triethylamine to react to prepare organic alkaline salt of bis(fluorosulfonyl)amine; performing replacing reaction with strong acid, and performing pressure-relief distillation, so as to obtain high-puritybis(fluorosulfonyl)amine. The preparation method has the advantages that the SO2F2, the NH4F and the organic alkaline are used as the raw materials, and the FSO3H (fluorosulfonic acid) with strong corrosion property is not used as the raw material; the technology is simple, the continuous operation property is strong, the product is easy to separate and purify, the high-purity (more than or equalto 99%) bis(fluorosulfonyl)amine can be prepared, and the yield rate reaches 90% or above; the chloride ion is not used in the whole reaction process, and the product quality is stable.
Owner:NANJING REDSUN BIOCHEM CO LTD +1
Fluorine and chlorine-containing conducting polymer resin preparation method
The invention discloses a fluorine and chlorine-containing conducting polymer resin preparation method. The fluorine and chlorine-containing conducting polymer resin preparation method comprises the following steps of: firstly mixing and blending a fluorine-containing liquid phase monomer with sulfuryl fluoride and polymerizable free radicals, purified water and a fluorine-containing surfactant to obtain prepolymer emulsion; then feeding a vapor-phase mixed monomer and a free radical initiator to carry out radical polymerization to obtain fluorine and chlorine-containing polymer emulsion with sulfuryl fluoride; and finally hydrolyzing the fluorine and chlorine-containing polymer emulsion with sulfuryl fluoride, condensing, washing and drying to obtain fluorine and chlorine-containing conducting polymer resin. The fluorine and chlorine-containing conducting polymer resin obtained by the preparation method has excellent physical and chemical properties such as relatively mechanical strength, water resistance, water vapor permeability, oil resistance, washing resistance, flame resistance, biochemical weapon penetration resistance and high conductivity.
Owner:ZHEJIANG HYPROOF TECH CO LTD +1
Preparation method of imidodisulfuryl fluoride lithium salt
ActiveCN107215853AEasy to operateMild conditionsNitrosyl chlorideLi-accumulatorsHydrogen fluorideFluoride
The invention relates to a preparation method of imidodisulfuryl fluoride lithium salt. The preparation method comprises the following steps: (1) performing a fluorination reaction of imidodisulfuryl chloride by taking a hydrogen fluoride complex salt as a fluorinating agent in the presence of a solvent, the fluorinating agent and an initiator, performing filter pressing separation at the end of the reaction, distilling filtrate under a reduced pressure to remove the solvent, and rectifying under a reduced pressure to obtain imidodisulfuryl fluoride; (2) reacting the imidodisulfuryl fluoride with a lithium compound at the temperature of 80 to 110 DEG C to obtain a crude imidodisulfuryl fluoride lithium salt. The preparation method provided by the invention has the advantages of easiness in operation of a fluorinating process route, mild conditions, high operability and easiness in implementation of industrial production; the prepared imidodisulfuryl fluoride lithium salt is 99 percent or more in purity, and is suitable for lithium batteries.
Owner:SUZHOU HUAYI NEW ENERGY TECH CO LTD
Process for preparing high-purity trifluoromethyl sulphonic acid
ActiveCN101402591AFast hydrolysisAdequate responseElectrolysis componentsOrganic compound preparationAlkaline earth metalDistillation
The invention relates to a process method for preparing high-purity trifluoromethane sulfonic acid. The process method comprises the following steps: firstly, hydrolyzing trifluoromethane sulfuryl fluoride gas prepared by an electrochemical fluorination method in alkaline metal or alkaline-earth metal hydroxide solution, recrystallizing the generated trifluoromethane sulphonate in a solvent to purify, and then reacting the trifluoromethane sulphonate with 100 percent of sulfuric acid in the presence of silicon dioxide to obtain an initial product of the trifluoromethane sulfonic acid, and finally purifying the trifluoromethane sulfonic acid through reduced pressure distillation. The process method not only effectively improves the hydrolysis speed of the trifluoromethane sulfuryl fluoride gas, leads the trifluoromethane sulfuryl fluoride gas to react more completely, and improves the yield, but also effectively reduces the content of F<-> in the trifluoromethane sulfonic acid product, and improves the purity of the product.
Owner:PERIC SPECIAL GASES CO LTD
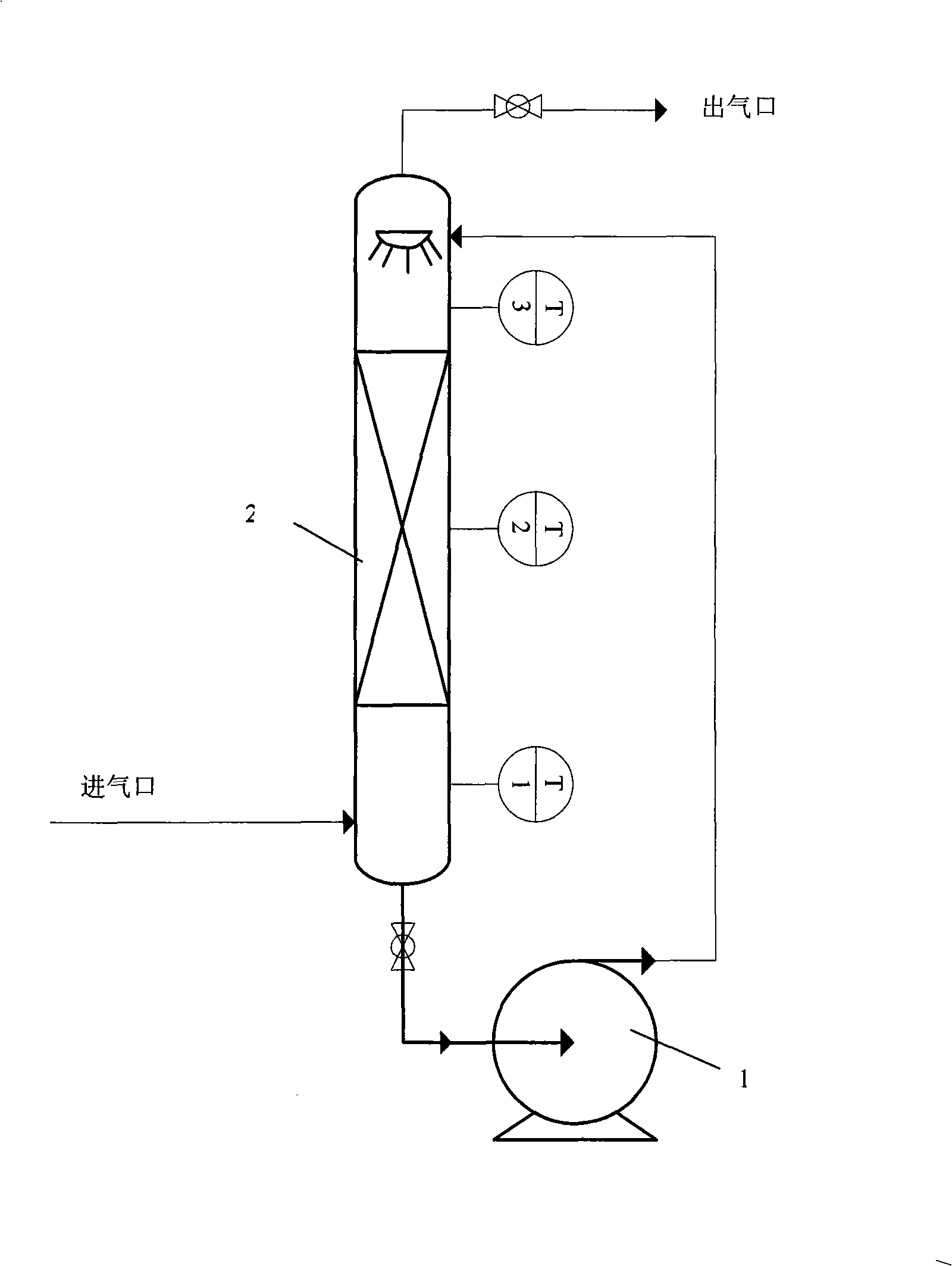
Method for removing sulfuryl fluoride by coupling plasma and chemical absorbing
ActiveCN102512926AProtect your healthSimple processDispersed particle separationPhysical well beingHuman health
The invention belongs to the technical field of gas pollutant treatment and provides a method for removing sulfuryl fluoride by coupling plasma and chemical absorbing, which is used for removing residual sulfuryl fluoride gas after fumigating. The treatment process of the method comprises: introducing a gas containing sulfuryl fluoride into a plasma reactor, wherein the sulfuryl fluoride decomposes into harmful gas products including sulfur oxide and fluoride under the action of the plasma; and introducing the gas product of the reaction into a gas liquid absorbing tower to absorb the gas by alkali liquor, and thus removing the sulfuryl fluoride waste gas in a harmless manner. The method has the advantage that after the sulfuryl fluoride is removed by coupling a plasma technique and chemical absorption, the treated gas reaches national discharge standards. Compared with discharging the sulfuryl fluoride into the atmosphere through ventilation at present, the method protects natural environment and human health. In addition, with simple process flow and low operation cost, the method is more suitable for on-site use.
Owner:ZHEJIANG UNIV OF TECH
Process for production of fluoro derivative
InactiveUS7807858B2Improve solubilityEfficient removalSugar derivativesOrganic compound preparationHydrogen fluorideOrganic base
It was found that a fluoro derivative can be produced by reacting a hydroxy derivative with sulfuryl fluoride (SO2F2) in the presence of an organic base or in the presence of an organic base and “a salt or complex comprising an organic base and hydrogen fluoride”. According to the present production process, it is not necessary to use perfluoroalkanesulfonyl fluoride, which is not preferable in industrial use, and it is possible to advantageously produce optically-active fluoro derivatives, which are important intermediates of medicines, agricultural chemicals and optical materials, specifically 4-fluoroproline derivatives, 2′-deoxy-2′-fluorouridine derivatives, optically-active α-fluorocarboxylate derivatives, and the like, even in a large scale.
Owner:CENT GLASS CO LTD
Method for preparing perfluorohexane surfactant serving as main agent of aqueous film-forming extinguishing agent directly
The invention discloses a method for preparing a perfluorohexane surfactant serving as a main agent of an aqueous film-forming extinguishing agent directly. The method comprises the following steps of: performing ammonolysis reaction of perfluorohexane sulfuryl fluoride and diamine which serve as raw materials under the action of a catalyst to synthesize an amino sulfonamide compound without separation, and performing quaterisation reaction of the amino sulfonamide compound and chloroacetate to prepare a perfluorohexane betaine amphoteric surfactant which can be used for the aqueous film-forming extinguishing agent directly. The method has the characteristics of simple requirement on equipment, stable and convenient process, low energy consumption and no pollution; and the aqueous film-forming extinguishing agent prepared from the perfluorohexane betaine amphoteric surfactant serving as the main agent is short in fire control time and high in fire extinguishing efficiency, and all indexes reach the national standards of the industry.
Owner:徐衡
Fumigant for wood parasitic nematode and method of wood fumigation
The invention provides a wood parasitic nematode fumigant, which is characterized in that methyl iodide dissolved in liquefied carbon dioxide gas is used instead of destructive matters from an ozonosphere, to use bromomethane limited in the world range; or, the methyl iodide is used together with any, or two or more compound of the sulfuryl fluoride, the methyl isothiocyanate, the phosphine, the ethylene oxide, the carbonyl sulfide and the propylene oxide; the invention also provides a wood fumigating method, which can kill the wood parasitic nematode by using the fumigant.
Owner:SUMIKA GREEN +2
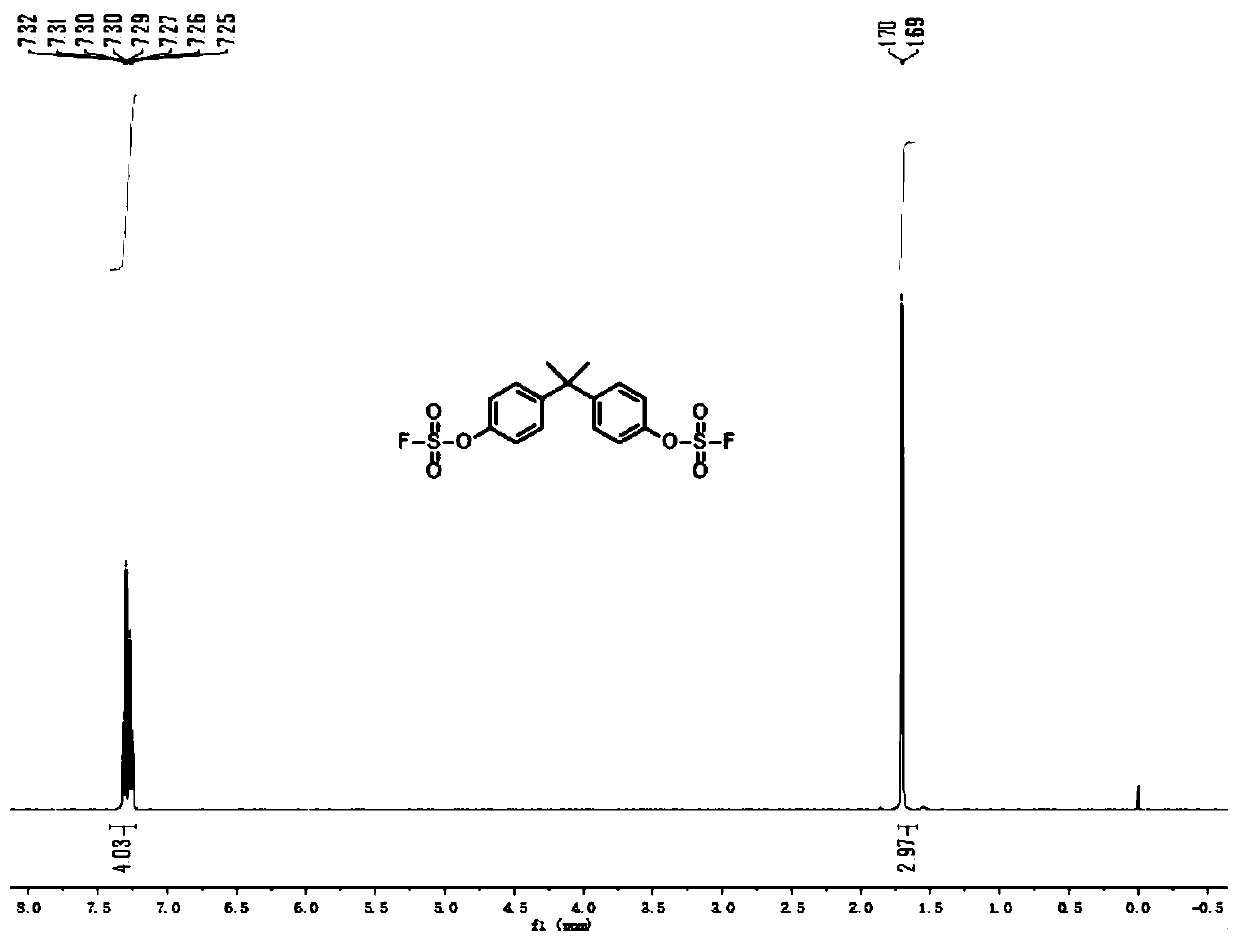
Sulfate preparation method
The invention relates to the field of organic synthesis, and especially relates to a sulfate preparation method. The invention provides a sulfate preparation method. According to the preparation method, a compound represented by a formula (II) reacts with sulfuryl fluoride in the presence of a reaction solvent to prepare a compound represented by a formula (I). During the sulfate preparation process, the introduction of water and chlorine ions is avoided effectively, the situation that the product is degraded and the chlorine ion content is high is avoided therefore; moreover, the steps of thepreparation method are simple and short, the raw material are common chemical products on the market, the kinds of raw materials and the side reactions are few, and the yield is high. The manufacturing cost is low, only recyclable organic solvents are used, the reaction byproduct is a single inorganic salt solid and can be easily recovered, no wastewater is generated, the environment is better protected, and the sulfate preparation method is green and environmentally friendly and is suitable for industrial large scale production.
Owner:SHANGHAI CHEMSPEC CORP +1
Preparation method of fasudil hydrochloride
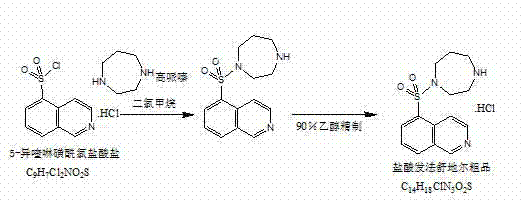
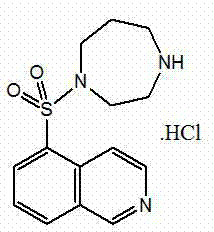
InactiveCN103044403ASolve problems that are difficult to obtain through suction filtrationReduce stepsOrganic chemistryIsoquinolineKinase
The invention belongs to the technical field of medicine, and particularly relates to a preparation method of a protein kinase inhibitor fasudil hydrochloride. According to the method, as 5-isoquinoline sulfuryl chloride hydrochloride is adopted to react with homopiperzine directly, the problem that 5-5-isoquinoline sulfuryl chloride is easy to hydrolyze is avoided; and as fasudil hydrochloride is obtained by a direct water phase evaporating method, the problem that the extraction filtration of fasudil hydrochloride is difficult is solved. The preparation method is simple to operate, and higher in product yield and product purity.
Owner:成都天翼医药科技有限公司
Chlorinating agents
InactiveUS20150057471A1Less-costly to mixAcceptable reaction ratePreparation by hydrogen halide split-offPreparation by halogen additionSulfuryl fluorideSulfuryl chloride
Owner:BLUE CUBE IP
Gas chromatography device for detecting sulfuryl fluoride in sulfur hexafluoride decomposition product
The invention discloses a gas chromatography device for detecting sulfuryl fluoride in a sulfur hexafluoride decomposition product. The gas chromatography device comprises a sulfur hexafluoride separation device, a capillary column sampling device, a Gaspro chromatographic column, a micro-plate flow path control valve and a second sulfide detector, wherein the capillary column sampling device comprises a first inlet, a second inlet and a first outlet which are communicated with one another; the second inlet is communicated with the sulfur hexafluoride separation device; auxiliary gas is fed through the first inlet; the micro-plate flow path control valve comprises a third inlet, a second outlet and a third outlet; the Gaspro chromatographic column is respectively communicated with the first outlet and the third inlet; the second sulfide detector is communicated with the second outlet. The gas chromatography device for detecting the sulfuryl fluoride in the sulfur hexafluoride decomposition product, provided by the invention, can be used for accurately detecting the sulfuryl fluoride.
Owner:STATE GRID CORP OF CHINA +1
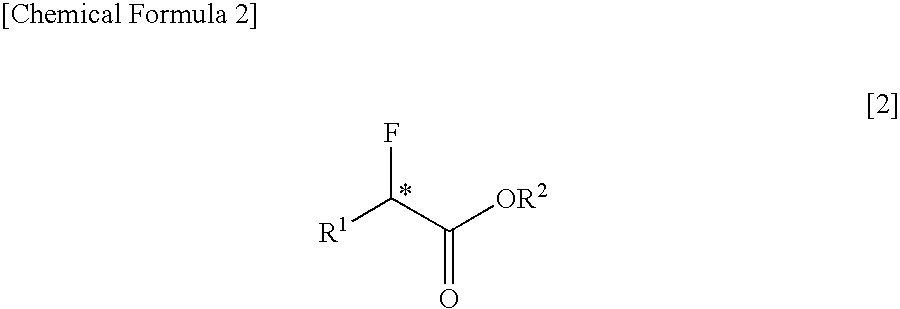
PROCESS FOR PRODUCING OPTICALLY ACTIVE alpha-FLUOROCARBOXYLATE ESTER
InactiveUS20100087673A1Promote recoveryHigh yieldOrganic compound preparationOrganic chemistry methodsOrganic baseDistillation
An optically active α-fluorocarboxylate is produced by reacting an optically active α-hydroxycarboxylate with sulfuryl fluoride (SO2F2), trifluoromethanesulfonyl fluoride (CF3SO2F) or nonafluorobutanesulfonyl fluoride (C4F9SO2F) in the presence of organic base and in the absence of reaction solvent. More preferably, a distillation purification is conducted after adding acid to the reaction-terminated liquid. With this, it is possible to produce an optically active α-fluorocarboxylate of a still higher purity. It is possible by this process to advantageously produce an optically active α-fluorocarboxylate on a large-amount scale.
Owner:CENT GLASS CO LTD
Ethylene sulfate preparation method
InactiveCN110818674AHigh purityChange cycle performanceOrganic chemistrySecondary cellsElectrolytic agentSulfate
The invention relates to an ethylene sulfate preparation method, which comprises: dissolving ethylene glycol in an aprotic organic solvent, adding an aprotic organic alkali, introducing sulfuryl fluoride, stirring while reacting, filtering after the reaction is completed, and carrying out evaporating concentrating and drying on the filtrate to obtain ethylene sulfate. According to the invention, the ethylene sulfate prepared by the method has extremely high purity of more than 99.9%, low moisture content of less than or equal to 20 ppm and low acid value of less than or equal to 10 ppm, and effectively changes the influence of the moisture and the acid value in an electrolytic solution on the cycle performance and the storage stability of a battery.
Owner:JIUJIANG TINCI ADVANCED MATERIALS CO LTD
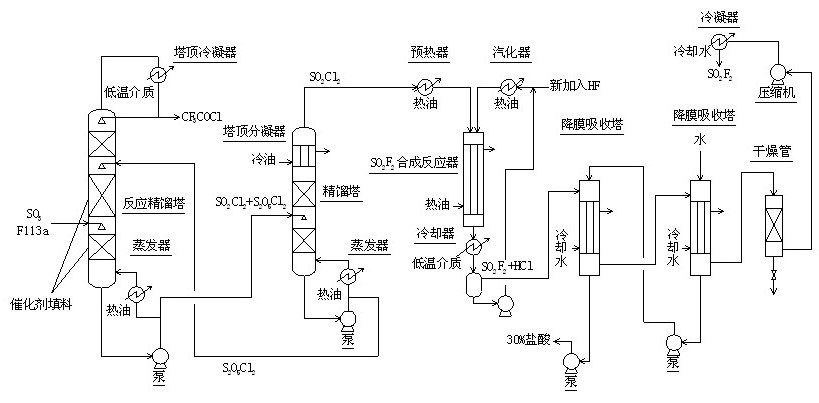
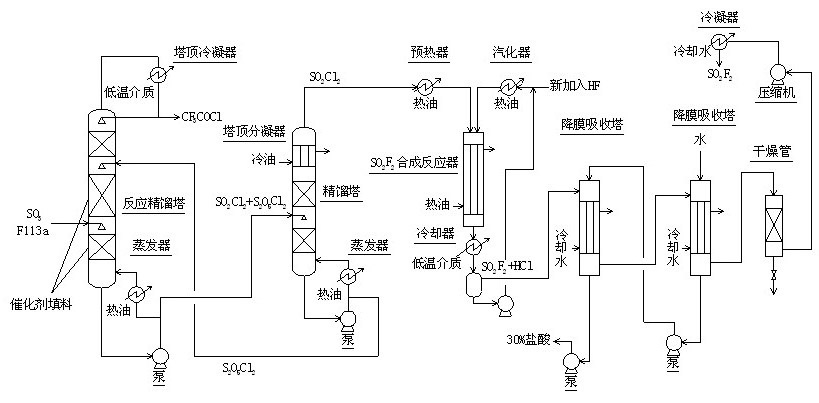
Method for continuously synthesizing trifluoroacetyl chloride and sulfuryl fluoride
InactiveCN102351681AHigh reaction yieldThree wastes lessSulfur-halogen-hydrogen-oxygen compoundsCarboxylic acid halides preparationReaction temperatureTrifluoroacetyl chloride
The invention relates to a method for continuously synthesizing trifluoroacetyl chloride and sulfuryl fluoride, which comprises the following process: continuously introducing sulfur trioxide and trifluorotrichloroethane (F113a) into a reaction rectifying tower containing a catalyst and a filler thorough the lower part in the tower according to a mol ratio of 1:1, wherein the temperature of the tower is controlled to range between 120 DEG C and 130 DEG C, and the reflux ratio at the top of the tower is 2.5-3; introducing tower bottoms into a sulfuryl chloride separating tower for rectification and separation, wherein the tower temperature is 145-150 DEG C, and the reflux ratio is 0.5-1.0; returning pyrosulfuryl chloride in the separating tower to the middle upper part of the reaction rectifying tower, heating the sulfuryl chloride rectified off from the top of the tower to 150 DEG C, and then introducing the rectified sulfuryl chloride and preheated recycled and newly-added hydrogen fluoride gas into a reactor containing a palladium / charcoal catalyst, wherein the reaction temperature is controlled to range between 150 DEG C and 160 DEG C; separating out unreacted hydrogen fluoride from the reaction product through a cold trap, and returning to the reactor for further use; and then absorbing and separating hydrogen chloride through a falling film, drying, compressing, and condensing to obtain the sulfuryl fluoride. The invention has the advantage of easy acquisition of raw materials, and the sulfuryl chloride byproduct can be directly used for the synthesis of sulfuryl fluoride.
Owner:ZHEJIANG UNIV +1
Preparation method and application of amphoteric fluorinion-containing ionic surfactant
InactiveCN102489216ARaw materials are easy to getEasy to prepareTransportation and packagingMixingSodium chloroacetateEvaporation
The invention discloses a preparation method and application of amphoteric fluorinion-containing ionic surfactant. The method comprises the following steps of: performing amidation on perfluoroalkyl sulfuryl fluoride and N, N'-dimethyl (diethyl)-1,3-propane diamine to obtain an intermediate 1, refining, performing amidation on a refined intermediate 1 and alkyl sulfuryl fluoride (chloride) or alkyl acyl fluoride (chloride) to obtain an intermediate 2, refining, and reacting the refined intermediate 2 and sodium chloroacetate and other substances to obtain the fluorinion-containing imine amphoteric ionic surfactant; and the surfactant can be used as emulsifier, foam stabilizer in a high-efficiency foam extinguishing agent, a wetting agent, a dispersing agent, a metal surface treatment agent, fire extinguishing agent additive, a foam flotation agent, and a crude oil evaporation inhibitor. The invention has the advantages that: the surfactant is low in production cost, and has low requirements on production equipment; the preparation method is simple; a product is environment-friendly, high in yield, excellent in surface property; the conversion rate of fluorinion-containing monomer reaches 96 percent; and the minimum surface tension of certain compounds on water is less than 17mN / m.
Owner:HUAZHONG NORMAL UNIV
Preparation method of fluorine-containing non-ionic surface active agent and application
ActiveCN103657514ARaw materials are easy to getEasy to prepareTransportation and packagingMixingEpoxyPropylenimine
The invention discloses a preparation method of a fluorine-containing non-ionic surface active agent and an application. The preparation method comprises the steps of carrying out amidation reaction on perfluoroalkyl sulfuryl fluoride and N, N'-dimethyl (ethyl)-1, 3-propane diamine to obtain an intermediate 1; after refining, adopting acetone as a solvent to react with ethylene oxide or chlorethyl alcohol to obtain an intermediate 2 (N'-3-(dimethyl(ethyl))-propyl-(N-perfluorobutylsulfonyl-N-epoxy)-amine); carrying out ring-opening reaction on the intermediate 2 and ethylene oxide according to different proportions to obtain the fluorine-containing non-ionic surface active agent. The preparation method disclosed by the invention has the advantages that the production cost of the surface active agent is low, the preparation method is simple, the requirement on production equipment is low, and the production process is simple; the yield is high, the fluorine-containing monomer conversion rate reaches 93%; the surface performance is excellent, the lowest surface tension of certain compound to water is below 20mN / m and the HLB (Hydrophile Lipophile Balance) value of the surface active agent is easily changed.
Owner:湖北美畅环保科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com