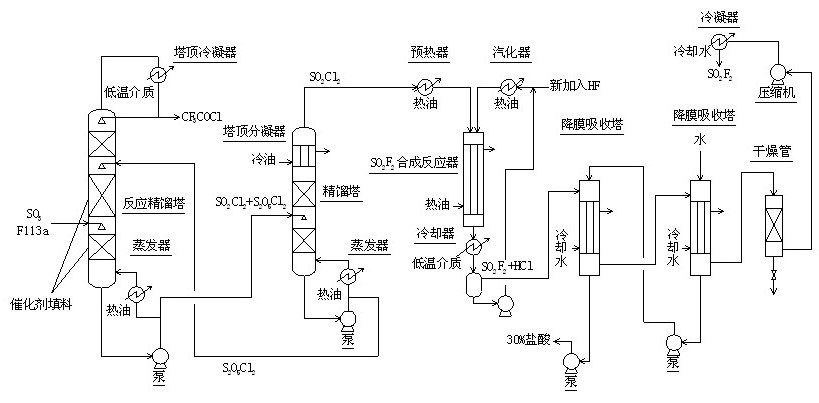
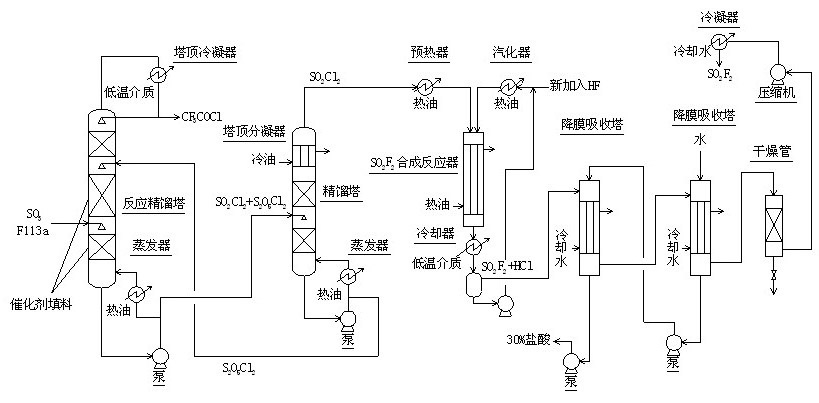
Method for continuously synthesizing trifluoroacetyl chloride and sulfuryl fluoride
A technology of trifluoroacetyl chloride and sulfuryl fluoride, which is applied in the preparation of acyl halides, sulfur-halogen-hydrogen-oxygen compounds, organic chemistry, etc., can solve the problem of high reaction temperature, high production cost, and no consideration of the separation and utilization of sulfuryl chloride problems and other problems, to achieve the effect of high reaction yield and less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1) Continuously pass 600 mol / h of sulfur trioxide and 600 mol / h of trifluorotrichloroethane (F113a) into the reactive distillation tower (diameter 50 mm, total height of 2000) at a distance of 500 mm from the bottom of the tower Mm, the middle and lower part of the tower are filled with fluorine sulfonic acid resin packing that has exchanged mercury salt and mercurous salt, and the upper 500 mm is filled with glass spring packing.) For reaction, control the temperature of the tower kettle to 120°C, The reflux ratio is 2.5, and the amount of trifluoroacetyl chloride extracted from the top of the tower is 594 moles / hour;
[0057] 2) The sulfuryl chloride and pyrosulfuryl chloride in the reactor of the reactive distillation tower are pumped into the sulfuryl chloride separation tower (50 mm in diameter, 600 mm in total height) at a distance of 200 mm from the bottom of the tower, and glass spring packing is installed inside. The packing is 400 mm.) Carry out rectification sep...
Embodiment 2
[0060] 1) The 600 mol / hour sulfur trioxide and 600 mol / hour trifluorotrichloroethane (F113a) are continuously fed into the reactive distillation tower (diameter 50 mm, total height of 2000) at a distance of 500 mm from the bottom of the tower Mm, the middle and lower parts of the tower are filled with fluorine sulfonic acid resin packing that has been exchanged for mercury salt and mercurous salt, and the upper 500 mm is filled with glass spring packing.) The reaction is carried out and the temperature of the tower kettle is controlled to 130°C. The reflux ratio is 3.0, and the amount of trifluoroacetyl chloride extracted from the top of the tower is 597 moles / hour;
[0061] 2) The sulfuryl chloride and pyrosulfuryl chloride in the reactor of the reactive distillation tower are pumped into the sulfuryl chloride separation tower (50 mm in diameter and 600 mm in total height) at a distance of 200 mm from the bottom of the tower with a glass spring packing inside. The packing is 400...
Embodiment 3
[0064] 1) The 600 mol / hour sulfur trioxide and 600 mol / hour trifluorotrichloroethane (F113a) are continuously fed into the reactive distillation tower (diameter 50 mm, total height of 2000) at a distance of 500 mm from the bottom of the tower Mm, the middle and lower part of the tower are filled with fluorine sulfonic acid resin packing that has been exchanged for mercury salt and mercurous salt, and the upper 500 mm is filled with glass spring packing.) For the reaction, control the temperature of the tower kettle to 127℃, The reflux ratio is 2.8, and the amount of trifluoroacetyl chloride extracted from the top of the tower is 596 moles / hour;
[0065] 2) The sulfuryl chloride and pyrosulfuryl chloride in the reactor of the reactive distillation tower are pumped into the sulfuryl chloride separation tower (50 mm in diameter and 600 mm in total height) at a distance of 200 mm from the bottom of the tower with a glass spring packing inside. The packing is 400 mm.) Carry out rectif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com