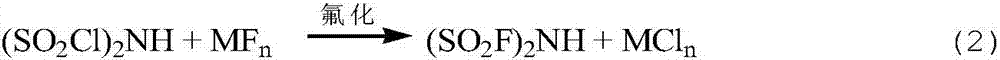Preparation method of bis(fluorosulfonyl)amine
A technology of bisfluorosulfonimide and ammonium fluoride, applied in the directions of nitrosyl chloride, nitrogen and non-metallic compounds, can solve the problems of high price of FSO3H, severe equipment corrosion, low yield, etc., and achieve easy separation, The effect of stable product quality and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 9g of NH to a 500mL autoclave 4 F (ACS grade), 100 g triethylamine and 210 g acetonitrile. Seal the reactor well and pump the reactor to a negative pressure of -0.1MPa (gauge pressure). With constant stirring, slowly introduce SO 2 f 2 The pressure of the gas to the reactor is 0.3MPa, and the reaction temperature is controlled at about 25°C. When the reaction pressure drops to 0.2MPa, continue to add sulfuryl fluoride to 0.3MPa, and cycle in turn until the pressure drops slowly or does not drop. Acyl fluoride no longer participated in the reaction, and the reaction was basically completed. The reaction was carried out for 20 hours in total, 60 g of sulfuryl fluoride was consumed, and 370 g of a light yellow transparent solution was obtained without solid residue. Acetonitrile and unreacted triethylamine were removed by rotary evaporation at 80°C and -0.08MPa (gauge pressure) conditions to obtain 145 g of dark yellow solution, then 30 g of concentrated sulfuric ac...
Embodiment 2
[0030] Add 9g of NH to a 500mL autoclave 4 F (ACS grade), 100 g triethylamine and 210 g acetonitrile. Seal the reactor well and pump the reactor to a negative pressure of -0.1MPa (gauge pressure). With constant stirring, slowly introduce SO 2 f 2 The gas keeps the pressure of the reactor at 0 MPa, the reaction temperature is kept at 25-28°C, the reaction is carried out for 24 hours, and 72g of SO is consumed 2 f 2 , 385 g of a light yellow transparent solution was obtained, and no solid remained. At 80 DEG C, under the condition of -0.08MPa (gauge pressure), rotary evaporation removes acetonitrile and unreacted triethylamine to obtain 138 g of dark yellow solution, then add 25 g of concentrated sulfuric acid (98% by mass), and the mixed solution is distilled under reduced pressure. 36.8g of HFSI product (purity 99.2%) was collected at 85°C / 650Pa as a colorless solution, which turned into white crystals after cooling with ice water, with a yield of 83.6%.
Embodiment 3
[0032] Add 9g of NH to a 500mL autoclave 4 F (ACS grade), 100 g triethylamine and 210 g acetonitrile. Seal the reactor well and pump the reactor to a negative pressure of -0.1MPa (gauge pressure). With constant stirring, slowly introduce SO 2 f 2 The pressure of the gas to the reactor is 0.6MPa, and the reaction temperature is controlled at about 25°C. When the reaction pressure drops to 0.2MPa, continue to add sulfuryl fluoride to 0.6MPa, and cycle in turn until the pressure drops slowly or does not drop, and the reaction After 14 hours, 75 g of sulfuryl fluoride was consumed to obtain 379 g of a light yellow transparent solution without solid residue. Acetonitrile and unreacted triethylamine were removed by rotary evaporation at 80°C and -0.08MPa (gauge pressure) conditions to obtain 150 g of dark yellow solution, then 30 g of concentrated sulfuric acid (98% by mass) was added, and the mixed solution was distilled under reduced pressure. 41.3g of HFSI product (purity 99....
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com