Preparation method of cyclic sulfate
A technology of cyclic sulfate and acid binding agent, applied in the direction of organic chemistry, etc., can solve the problems of low quality of crude product, increased cost of raw and auxiliary materials, difficulty in preparation of reagents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
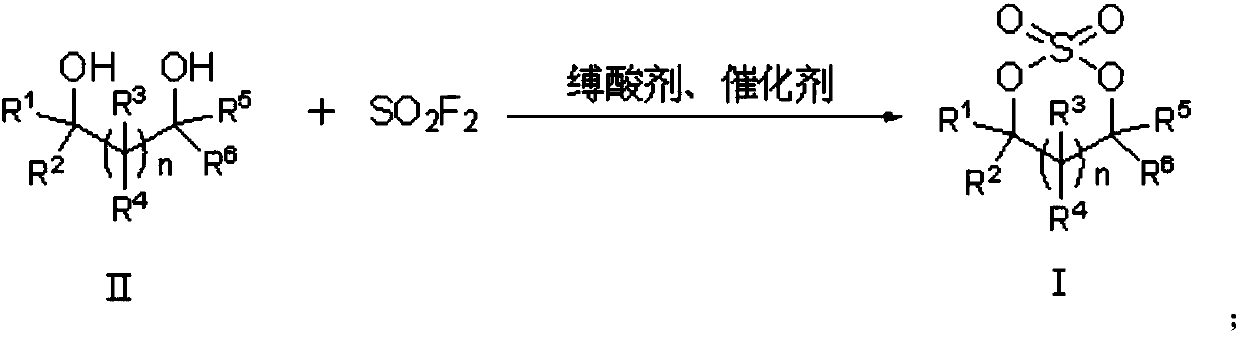
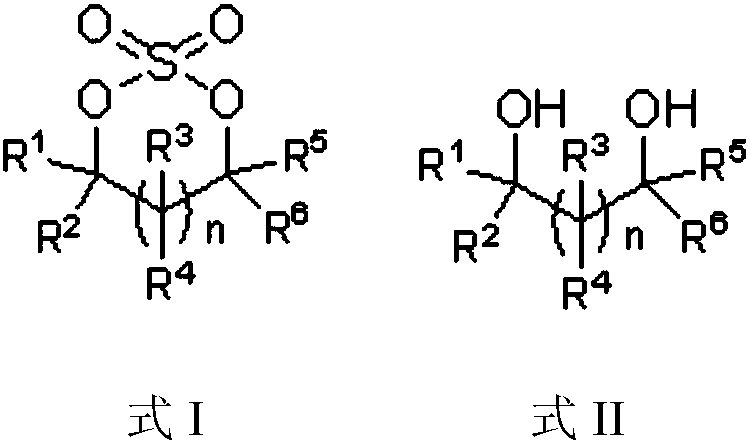
[0050]One aspect of the present invention provides a method for preparing a cyclic sulfuric acid ester. The structural formula of the cyclic sulfuric acid ester is shown in formula I, comprising the following steps: combining the compound of formula II with the presence of an acid-binding agent and a catalyst. Sulfuryl fluoride reaction is prepared to obtain the compound of formula I, and the reaction equation is as follows:
[0051]
[0052] In the preparation method of described cyclic sulfuric acid ester, n in formula I and / or formula II can be selected from 0, 1, 2, 3 or 4, thereby can constitute the corresponding number of carbon atoms in the formed formula I compound For ring structures, n can preferably be selected from 0 or 1.
[0053] In the preparation method of the cyclic sulfuric acid ester, R1, R2, R3, R4, R5, R6 can be independently selected from H, C1~C8 alkyl, aryl, C1~C8 alkoxy, halogen (for example, Fluorine, chlorine, bromine, iodine) or sulfate group, a...
Embodiment 1
[0072] The preparation of vinyl sulfate (formula III compound):
[0073]
[0074]Add 40g of ethylene glycol, 400mL of dichloromethane, 108g of solid potassium hydroxide, and 1.7g of tetra-n-butylammonium fluoride into a 1000mL reaction flask, keep the internal temperature at 0-5°C, and slowly introduce 98g of sulfuryl fluoride gas under stirring , reacted for 1 hour, blown nitrogen for 1 hour, filtered, and the filtrate was precipitated under reduced pressure to obtain a solid crude product. At room temperature, filter and dry to obtain 54.1 g of the product, with a yield of 67.6%. Test results: GC (%): >99.0%; AAS (ppm): Na 0.2, K 3, Fe 0.3, Ca 0.5; IC (ppm): SO 4 2- 49,F - 0.3, Cl - 0.1.
Embodiment 2
[0076] Preparation of vinyl sulfate:
[0077] In a 1000mL autoclave, add 40g of ethylene glycol, 250mL of methyl tert-butyl ether, 59g of solid sodium hydroxide, 20g of sodium sulfate, and 4.3g of tetra-n-butylammonium hydrogensulfate. 72 g of sulfuryl fluoride gas was introduced, and the reaction was sealed for 1 hour. After the reaction was completed, nitrogen was blown for 1 hour, filtered, and the filtrate was desolvated under reduced pressure to obtain a solid crude product. Add 100 mL of dichloromethane, 0.05 g of 15-crown-5, and 0.05 g of 18-crown-6, heat up and reflux to dissolve, and then slowly cool down to room temperature. After filtering and drying, 65.5 g of the product was obtained, with a yield of 81.8%. Test results: GC (%): >99.9%; AAS (ppm): Na 4, K 0.5, Fe 0.4, Ca 0.4; IC (ppm): SO 4 2- 55,F - 0.5, Cl - 0.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com