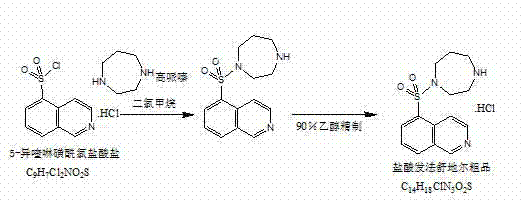
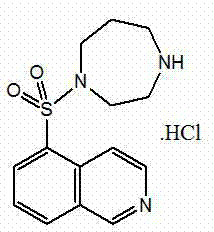
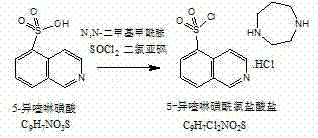Preparation method of fasudil hydrochloride
A technology of fasudil hydrochloride and isoquinolinesulfonyl chloride hydrochloride, which is applied in the field of medicine, can solve the problems that fasudil hydrochloride is difficult to pass through suction filtration, etc., so as to reduce the probability of acylation, the experimental operation is simple, and the reaction fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A preparation method of Fasudil hydrochloride, comprising the following steps:
[0046] (1) Preparation of 5-isoquinolinesulfonyl chloride hydrochloride
[0047]
[0048] Weigh 2250g of 5-isoquinolinesulfonic acid, add it to a 30L glass reaction kettle, add 18000ml of thionyl chloride, stir for 10min, add 450ml of N,N-dimethylformamide, stir for 10min, pass into the interlayer of the reaction kettle Heat conduction oil, stir and heat up to reflux, reflux reaction for 2 hours, after the reaction is completed, the reaction solution is evaporated to dryness under reduced pressure, 2000ml of dichloromethane is added to the reaction kettle, and the solvent is evaporated to dryness under reduced pressure, and then stirred with 10Kg of dichloromethane Wash 4 times, filter, and vacuum-dry the filter cake at 40°C for 4 hours to obtain a white solid, namely 2480.01 g of 5-isoquinolinesulfonyl chloride hydrochloride, yield: 87.31%.
[0049] (2) Preparation of Fasudil Hydrochlo...
Embodiment 2
[0056] (1) Preparation of 5-isoquinolinesulfonyl chloride hydrochloride
[0057] Weigh 2250g of 5-isoquinolinesulfonic acid, add it to a 30L glass reaction kettle, add 18000ml of thionyl chloride, stir for 10min, add 450ml of N,N-dimethylformamide, stir for 10min, pass into the interlayer of the reaction kettle Heat conduction oil, stir and heat up to reflux, reflux reaction for 1 hour, after the reaction is complete, evaporate the reaction solution to dryness under reduced pressure, add 2000ml of dichloromethane, then evaporate the solvent to dryness under reduced pressure, then add 10Kg of dichloromethane to stir wash twice, After filtration, the filter cake was vacuum-dried at 40°C for 4 hours to obtain a white solid, namely 2449.90 g of 5-isoquinolinesulfonyl chloride hydrochloride, yield: 86.25%.
[0058] (2) Preparation of Fasudil Hydrochloride Crude Product
[0059] Weigh 1706.48g of homopiperazine, add it into a glass reactor, add 30.4L of dichloromethane, stir and di...
Embodiment 3
[0063] (1) Preparation of 5-isoquinolinesulfonyl chloride hydrochloride
[0064] Weigh 2250g of 5-isoquinolinesulfonic acid, add it to a 30L glass reaction kettle, add 18000ml of thionyl chloride, stir for 10min, add 450ml of N,N-dimethylformamide, stir for 10min, pass into the interlayer of the reaction kettle Heat conduction oil, stir and heat up to reflux, reflux reaction for 5 hours, after the reaction is completed, the reaction solution is evaporated to dryness under reduced pressure, 2000ml of dichloromethane is added, and the solvent is evaporated to dryness under reduced pressure, then 10Kg of dichloromethane is added to stir wash 3 times, After filtration, the filter cake was vacuum-dried at 40°C for 4 hours to obtain a white solid, namely 2481.15 g of 5-isoquinolinesulfonyl chloride hydrochloride, yield: 87.35%.
[0065] (2) Preparation of Fasudil Hydrochloride Crude Product
[0066] Weigh 3412.96g of homopiperazine, put it into a glass reaction kettle, add 30.4L of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com