Patents
Literature
41results about How to "The reaction process is mild and easy to control" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
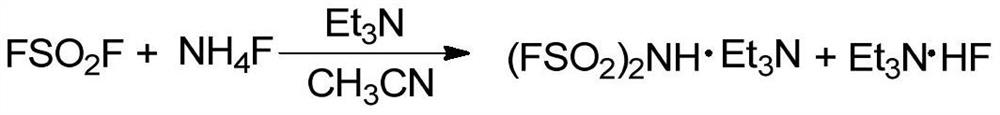
Preparation method of lithium bis (fluorosulfonyl) imide
PendingCN111620315AReduce usageThe reaction process is mild and easy to controlNitrosyl chlorideImideLithium oxide
The invention discloses a preparation method of lithium bis (fluorosulfonyl) imide. The preparation method comprises the following steps: (1) in the presence of an ammonia source, an organic solvent,fluoride salt and an initial amount of sulfuryl fluoride, slowly introducing organic alkali while continuously introducing the balance of sulfuryl fluoride until the reaction is finished, and directlycarrying out reduced pressure distillation on the reaction solution to obtain an intermediate, namely the difluorosulfonyl imide salt; and (2) in the presence of an organic solvent, adding lithium oxide powder into the intermediate imidodisulfuryl fluoride salt, filtering, concentrating, adding a non-aqueous poor solvent, and crystallizing to obtain lithium bis (fluorosulfonyl) imide.
Owner:SHANGHAI HUAYI GRP CO +1
Preparation method for hypophosphorous acid / phosphorous acid/ phosphate compounds by adopting P(O)-OH-contained compounds
ActiveCN103980306AThe reaction process is mild and easy to controlHigh yieldGroup 5/15 element organic compoundsPhosphinic AcidsPhosphate
The present invention provides a highly selective synthesis method for hypophosphorous acid / phosphorous acid / phosphate derivatives containing different substituted functional groups. In the present invention, base is adopted as a catalyst, compounds containing P(O)-OH and halogenated aliphatic hydrocarbons are adopted as reaction substrates, and an organic solvent is added into the reaction system. The method has advantages that the catalyst is cheap and easily obtained; reaction conditions are mild, safe and reliable; the selectivity of target product is close to 100%, and the yield is up to 90%. According to the present invention, the problems of low reaction selectivity, tedious reaction steps, low yields, needing reagents harmful to environment and the like in conventional methods for synthesizing hypophosphorous acid / phosphorous acid / phosphate compound are solved. The method has good industrial application prospects. The present invention also provides the corresponding hypophosphorous acid / phosphorous acid / phosphate derivatives containing different substituted functional groups.
Owner:HUNAN UNIV
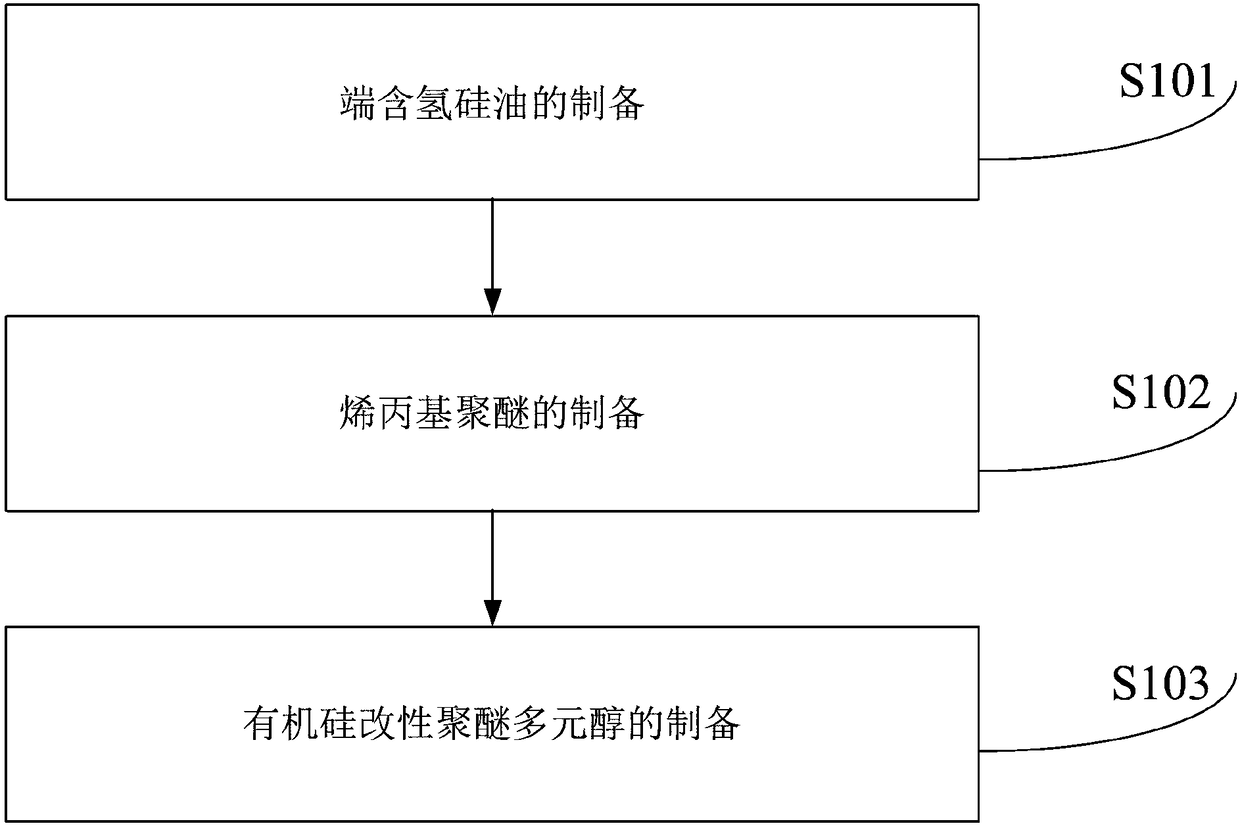
Preparation method of organic silicon modified polyether polyol
The invention provides a preparation method of organic silicon modified polyether polyol. The preparation method comprises following steps: step one, preparing terminal hydrogen containing silicone oil; step two, preparing allyl polyether; and step three, preparing organic silicon modified polyether polyol. The preparation method has the advantages that the structural characteristics of common polyether polyol are maximally preserved; the speed of reactions between modified polyether polyol and isocyanate is similar with that of reactions between common polyether polyol and isocyanate; modified polyether polyol can completely or partially replace common polyether polyol; modified polyether polyol can be used with polyester polyol to synthesize polyurethane, moreover, the reaction process is mild and easily controllable, the synthesized polyurethane is used to paint a material, the coated material is smooth and has a good wear resistant performance, and no surface migration happens.
Owner:江西三越新材料有限公司
Method for preparing organic lactone by catalyzing and oxidizing organic ketone with carbon materials
InactiveCN102603446AImprove stabilityEasy to makeCarboxylic acid ester formation/introductionCarbon nanotubeCatalytic oxidation
The invention provides a method for preparing organic lactone. The method is characterized in that carbon materials are used as catalyst, the carbon materials may be graphite, graphene, carbon nano tube, active carbon and so on; organic ketone is used as substrate, oxygen or air is used as oxygen source, organic aldehydes materials are used as reductant, and organic solvent is added into reaction system. The method has the following advantages: the preparation for the catalyst is simple; the reaction condition is mild, secure, credible and environmental friendly; the selectivity and the yield of the obtained target products are about 100%; and the catalyst can be used repeatedly. The method solves the problem that the traditional preparation technology for lactone has poor security and low yield, and has good industrial application prospect.
Owner:HUNAN UNIV
Method for preparing organic lactone
InactiveCN102603447AImprove stabilityEasy to makeCarboxylic acid ester formation/introductionMetal/metal-oxides/metal-hydroxide catalystsOrganic solventCobalt(II,III) oxide
The invention provides a method for preparing organic lactone. The method is characterized in that: cubic cobaltosic oxide is taken as a catalyst, and the catalyst is prepared with a hydrothermal method; and organic ketone is taken as a substrate, oxygen or air is taken as an oxygen source, an organic lactone substance is taken as a reducing agent, and an organic solvent is added into a reaction system. The method has the advantages that: the catalyst is easy to prepare; reaction conditions are mild, and the method is safe, reliable and environmentally-friendly; the selectivity and yield of an obtained target product are close to 100 percent; and the catalyst can be used for repeatedly. Due to the adoption of the method, the problems of poor safety, low yield and the like existing in the conventional lactone preparation process are solved, and the method has a good industrial application prospect.
Owner:HUNAN UNIV
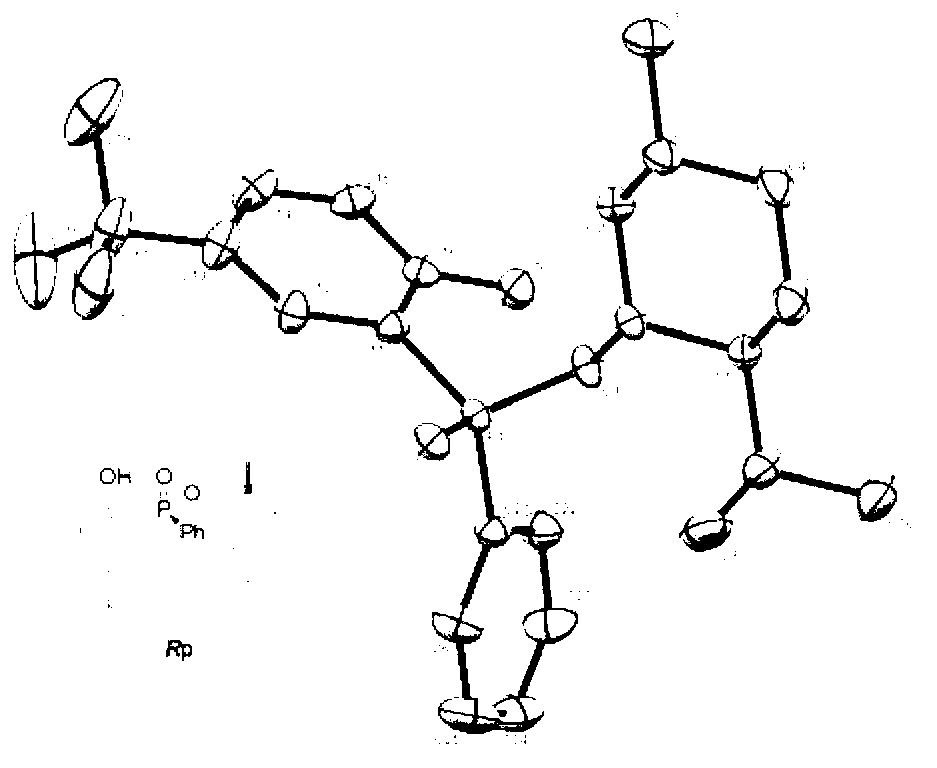
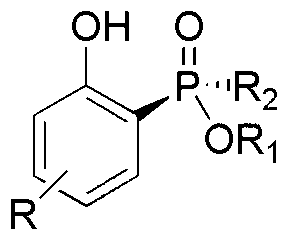
Phenol derivative containing (Rp)-2-chiral phosphinate substituent and preparation method thereof
ActiveCN103319526AHigh stereoselectivityHigh yieldGroup 5/15 element organic compoundsOrganic solventPhenol derivative
The invention provides a method for synthesizing a phenol derivative having a phosphorous chiral center and containing a (Rp)-2-chiral phosphinate substituent in a high stereoselectivity manner. According to the method, with small organic molecules as a catalyst and a (Rp)-2-chiral phosphinate compound containing P-H bonds, and phenol as reaction substrates, an organic solvent is added into a reaction system. The method has the advantages that the catalyst is low in cost and easy to obtain; reaction conditions are mild, safe and reliable; the stereoselectivity of the obtained target product is close to 100 percent and the yield of the obtained target product is up to 90 percent. According to the method provided by the invention, the defects of poor stereoscopic enantioselectivity, fussy reaction steps, low yield and the like of an organic phosphine compound containing the phosphorous chiral center, which is synthesized by adopting a traditional method, are overcome, and the method has a favorable industrial prospect. The invention further provides the phenol derivative having the phosphorous chiral center and containing the (Rp)-2-chiral phosphine substituent.
Owner:HUNAN UNIV
Method for preparing alpha-acyloxy ketone compound from terminal alkyne compounds
InactiveCN107011162AThe reaction process is mild and easy to controlHigh yieldOrganic compound preparationCarboxylic acid esters preparationOrganic solventAntioxidant
The invention provides a method for efficiently synthesizing an alpha-acyloxy ketone compound containing different substitute functional groups. According to the method, acetylacetone copper is taken as a reaction catalyst, t-butylhydroperoxide is taken as an antioxidant, ketone compounds and terminal alkyne compounds are taken as reaction substrates, and an organic solvent is added into a reaction system. The method has the advantages that the catalyst is cheap and easily available; reaction conditions are mild, safe and reliable; and the productivity of an obtained target product is relatively high. According to the method, the problem that a traditional alpha-acyloxylation reaction system is single is solved, and a novel acyloxylation reagent is provided; and the method has good industrial application prospects.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
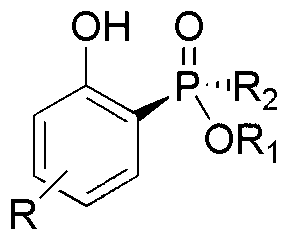
Phenol derivatives containing (Sp)-2-chiral phosphinate substituent groups and preparation method thereof
ActiveCN103304598AHigh yieldThe reaction process is mild and easy to controlGroup 5/15 element organic compoundsOrganic solventPhosphine
The invention provides a method for synthesizing phenol derivatives containing (Sp)-2-chiral phosphinate substituent groups with a phosphorus chiral center at high stereoselectivity. The method is characterized in that an organic small molecule material is taken as a catalyst, (Rp)-chiral phosphinate compounds containing a P-H bond and 2-halogenated phenol (-I, -Br) are taken as reaction substrates, and a base and an organic solvent are added to a reaction system. The method provided by the invention has the following advantages that the catalyst is cheap and easy to obtain, the reaction conditions are mild, and safe and reliable, the stereoselectivity of the obtained target product is close to 100% and the yield of the product is high; and the method overcomes the shortcomings of poor stereo enantioselectivity, complex reaction steps, low yield and the like of the traditional method for synthesizing a chiral phosphine compound with the phosphorus chiral center, and has an excellent industrial application prospect. The invention also provides phenol derivatives containing the (Sp)-2-chiral phosphinate substituent groups with the phosphorus chiral center.
Owner:HUNAN UNIV
Preparation method of N-phenyl maleimide heat-resistant modifier
ActiveCN104211848ASimple processThe reaction process is mild and easy to controlMaleic anhydrideSolvent
The invention discloses a preparation method of an N-phenyl maleimide heat-resistant modifier. The preparation method comprises the following steps: adding styrene, maleic anhydride, azodiisobutyronitrile, trithio dimethyl carbonate and 2-methyl-dithiobenzimidazole benzyl ester to form a polymerization system; carrying out a polymerization reaction for 2-6 hours at 60-120 DEG C under nitrogen protection; then, cooling to 60 DEG C, adding aniline, acetic anhydride and sodium acetate, and raising the temperature to 100-150 DEG C to react for 2-6 hours; and finally, cooling to room temperature, pouring the reactants into ethanol to settle a polymer, and filtering and drying to obtain a finished product. Through the above manner, the molecular weight and distribution of the product of the polymerization reaction can be conveniently controlled. The molecular weight of the prepared polymer is distributed below 1.3. Comparatively speaking, the molecular weight distribution is relatively narrow, the process is relatively simple, and the N-phenyl maleimide heat-resistant modifier has a relatively high economic value.
Owner:江苏科利新材料有限公司
Novel method for preparing diarylmethyl substituted phosphonate
ActiveCN112010898AHigh selectivityHigh yieldGroup 5/15 element organic compoundsChemical recyclingPhosphorous acidPtru catalyst
The invention provides a method for efficiently and highly selectively synthesizing diarylmethylphosphonate derivatives containing different substituted functional groups. According to the method, silver tetrafluoroborate is used as a catalyst, trialkyl phosphite and a 4-arylmethylene-2,6-di-tert-butyl-2,5-cyclohexadien-1-one compound are used as reaction substrates, and an organic solvent is added into a reaction system. The method has the advantages that the catalyst is cheap and easily available; substrate applicability is high; reaction conditions are mild, safe and reliable; the selectivity of the obtained target products is close to 100%; and the yield of the target products is as high as 90% or above. The method overcomes the defects of poor reaction selectivity, complex reaction steps, low yield, need for reagents harmful to the environment and the like in traditional synthesis of diarylmethyl substituted phosphonate derivatives, and has favorable industrial application prospects. The invention also provides the corresponding diarylmethyl substituted phosphonate derivatives containing different substituted functional groups at the same time.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
Method using aryl diazonium tetrafluoroborate and nitrile to prepare organic amide compounds
ActiveCN108069868AHigh selectivityThe reaction process is mild and easy to controlOrganic compound preparationOrganic chemistry methodsHigh selectivityOrganic compound
The method provides a method for efficiently synthesize organic amide compounds containing different substitution functional groups in a high selectivity manner. The method is characterized in that copper iodide is used as the catalyst, aryl diazonium tetrafluoroborate compounds and organic nitrile compounds are used as the reaction substrates, and an organic solvent, water and alkali are added into the reaction system. The method has the advantages that the catalyst is cheap and easy to obtain; the substrates are high in adaptability; reaction conditions are mild, safe and reliable; the selectivity of the obtained target product is close to 100%, and the yield of the target product reaches up to 90% or above; defects that a traditional method for synthesizing the organic amide compounds is harsh in reaction condition, poor in reaction selectivity, complex in experiment steps and low in yield, needs reagents harmful to environments and the like are overcome, and the method is promisingin industrial application prospect. The invention further provides the corresponding organic amide compounds containing different substitution functional groups.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
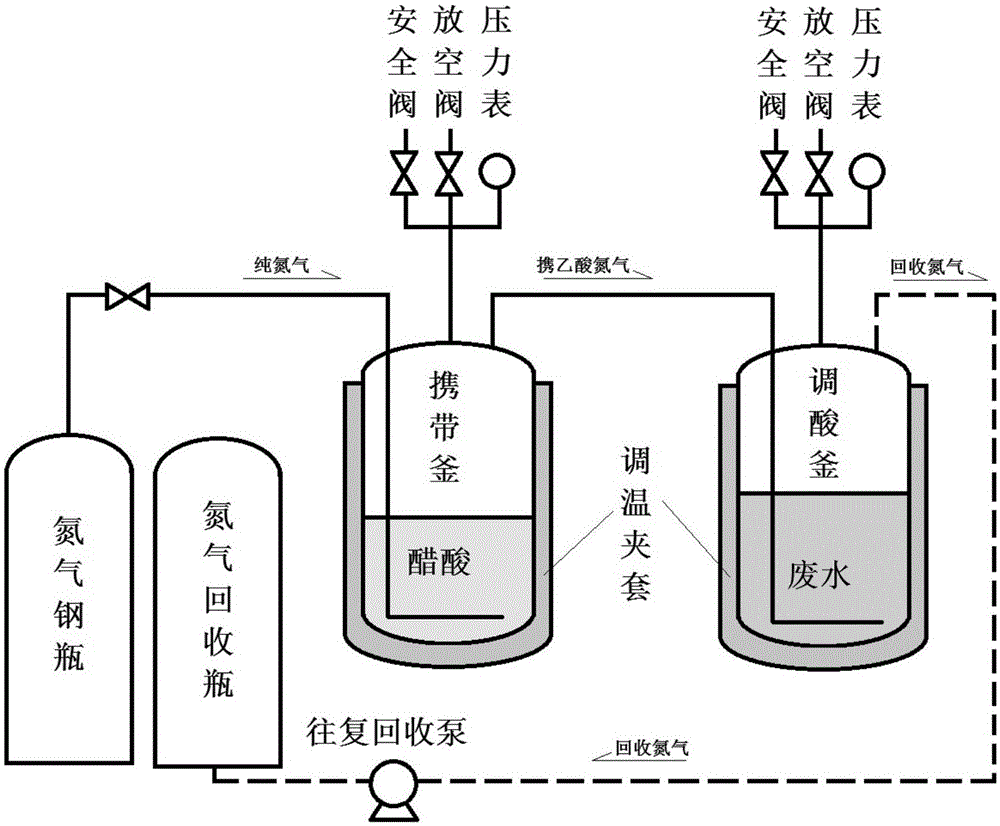
Recovery method of protein in high concentration protein wastewater
InactiveCN106007107AEfficient removalHigh removal rateWater/sewage treatment by centrifugal separationWater treatment parameter controlAcetic acidHigh concentration
The invention provides a recovery method of protein in high concentration protein wastewater. Nitrogen circulation carries acetic acid into high concentration protein wastewater to adjust pH change step by step to different protein isoelectric points (pI), thus achieving graded isoelectric precipitation and recovery of protein from wastewater. The method provided by the invention adopts carry of acetic acid by pressurized nitrogen circulation as an acidic adjustment means, makes the pH value adjustment mild and controllable, can precipitate out proteins with different isoelectric points under different operation conditions respectively, thereby achieving the purpose of efficient and graded removal of protein from high concentration protein wastewater by protein isoelectric point precipitation technique.
Owner:OCEAN UNIV OF CHINA
Method for preparing organic phosphate compounds from P(O)-OH compounds and methyl-containing substituted aromatic hydrocarbons
ActiveCN107602609AHigh selectivityEfficient synthesisGroup 5/15 element organic compoundsOrganic solventPhosphate
The invention provides a high-efficiency and high-selectivity synthetic method for organic phosphate derivatives containing different substituted functional groups. The method uses tetrabutylammoniumiodide as a catalyst and uses a P(O)-OH-containing compound and a methyl-containing aromatic hydrocarbon compound as reaction substrates, and an organic solvent and an oxidant are added in the reaction system. The method has the advantages that the catalyst is cheap and easy to obtain, the substrate applicability is high, the reaction conditions are mild, safe and reliable, the selectivity of an obtained target product is close to 100%, and the yield is up to 90% or more. The method solves shortages in traditional organic phosphate ester compound synthetic methods that reaction selectivity ispoor, reaction steps are complicated, the yield is low, environmentally-harmful agents are used and the like, and has better industrial application prospects. The invention also provides the corresponding organic phosphate derivatives containing the different substituted functional groups.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
Method for preparing organic lactone by catalyzing and oxidizing organic ketone with carbon materials
InactiveCN102603446BImprove stabilityEasy to makeCarboxylic acid ester formation/introductionCatalytic oxidationCarbon nanotube
The invention provides a method for preparing organic lactone. The method is characterized in that carbon materials are used as catalyst, the carbon materials may be graphite, graphene, carbon nano tube, active carbon and so on; organic ketone is used as substrate, oxygen or air is used as oxygen source, organic aldehydes materials are used as reductant, and organic solvent is added into reaction system. The method has the following advantages: the preparation for the catalyst is simple; the reaction condition is mild, secure, credible and environmental friendly; the selectivity and the yield of the obtained target products are about 100%; and the catalyst can be used repeatedly. The method solves the problem that the traditional preparation technology for lactone has poor security and low yield, and has good industrial application prospect.
Owner:HUNAN UNIV
Novel green method for preparing 2-diarylmethyl substituted-1-naphthol compound
PendingCN114369011AHigh selectivityHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationArylO-Phosphoric Acid
The invention provides a method for efficiently and highly selectively synthesizing 2-diarylmethyl substituted-1-naphthol compounds containing different substituted functional groups, which adopts phosphoric acid as a catalyst, adopts 1-naphthol compounds and 4-arylmethylene-2, 6-dialkyl-2, 5-cyclohexadiene-1-ketone compounds as reaction substrates, and adopts a one-pot method to synthesize the 2-diarylmethyl substituted-1-naphthol compounds containing different substituted functional groups. The reaction system takes water as a solvent. The method has the advantages that the catalyst is cheap and easily available; the substrate applicability is high; reaction conditions are mild, safe and reliable; the selectivity of the obtained target product is close to 100%, and the yield is high. The method overcomes the defects of poor reaction selectivity, tedious reaction steps, low yield, need of reagents harmful to the environment and the like in traditional synthesis of the 2-diarylmethyl substituted-1-naphthol compound, and has a good industrial application prospect. The invention also provides the corresponding 2-diarylmethyl substituted-1-naphthol compounds containing different substituted functional groups at the same time.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
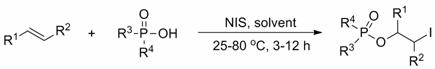
Method for preparing 2-iodo-1-phosphoryl substituted alkane compound through efficient olefin bifunctionalization
The invention provides a method for efficiently and highly selectively synthesizing a substituted 2-iodo-1-phosphoryl substituted alkane compound containing different functional groups. N-iodosuccinimide (NIS) is used as an accelerant, a compound containing P(O)-OH and olefin are used as reaction substrates, and an organic solvent is added into a reaction system. The method has the advantages thatthe accelerant is cheap and easy to obtain; the substrate applicability is high; reaction conditions are mild, safe and reliable; the selectivity of the obtained target product is close to 100%; andthe yield is as high as 90% or above. According to the method, a new way for synthesizing the substituted 2-iodo-1-phosphoryl substituted alkane compound containing different functional groups is developed, the defects of poor reaction selectivity, complex reaction steps, low yield, need of reagents harmful to the environment and the like in traditional synthesis of the 2-iodo-1-phosphoryl substituted alkane compound are overcome, and the method has a good industrial application prospect. The invention also provides a corresponding substituted 2-iodo-1-phosphoryl substituted alkane derivativecontaining different functional groups.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
Method for preparing C2-position alkynyl compound by catalyzing thiamine type compound desulfuration through cuprous halides
InactiveCN105732245AHigh yieldThe reaction process is mild and easy to controlOrganic chemistry methodsThiamineOrganic solvent
The invention provides a method for efficiently synthesizing derivatives which contain different substitution functional groups and have C2-position alkynyl substituted.According to the method, Pd(OAc)2(PPh3)2 / CuI is used as a catalyst, Na2CO3 is added as alkaline, compounds containing thiamine and terminal alkyne are used as oligomer, and organic solvent is added to the reaction system.The method has the advantages that the catalyst is low in price and easy to obtain; the reaction condition is mild, safe and reliable; the highest yield of target products reaches 92%.The method overcomes the defects that in traditional desulfuration reaction, cuprous thiophene needs to be used as a desulfurizing agent and cuprous halides can not react; the operation process is simple, and good industrial application prospects are achieved.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
A new method for preparing 2'-iodo[1,1'-biaryl]-2-organophosphonate compounds
The invention provides a new method for efficiently and highly selectively synthesizing 2'-iodo[1,1'-biaryl]-2-organophosphonate compounds containing different functional groups, which uses cheap transition metal copper The salt is used as a catalyst, and the compound containing P(O)-OH and bisaryltrifluoromethanesulfonic acid periodiodium salt is used as a reaction substrate, and an organic solvent and a base are added to the reaction system. The advantages of the method are: the catalyst is cheap and easy to obtain; the substrate has high applicability; the reaction conditions are mild, safe and reliable; the selectivity of the obtained target product is close to 100%, and the yield is as high as more than 90%. The method solves the shortcomings of the traditional synthesis of 2'-iodo[1,1'-biaryl]-2-organophosphonate compounds, such as poor reaction selectivity, cumbersome reaction steps, low yield, and the need to use environmentally harmful reagents. , has good industrial application prospects. The present invention also provides corresponding 2'-iodo[1,1'-biaryl]-2-organophosphonate derivatives substituted with different functional groups.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
A New Method for the Efficient Preparation of Chiral Organic Phosphonates Containing r-/s-Diarylmethyl Substitutions by Chiral Induction
ActiveCN109503656BWays to avoid chiral resolutionHigh stereoselectivityGroup 5/15 element organic compoundsOrganic chemistry methodsPtru catalystOrganosolv
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
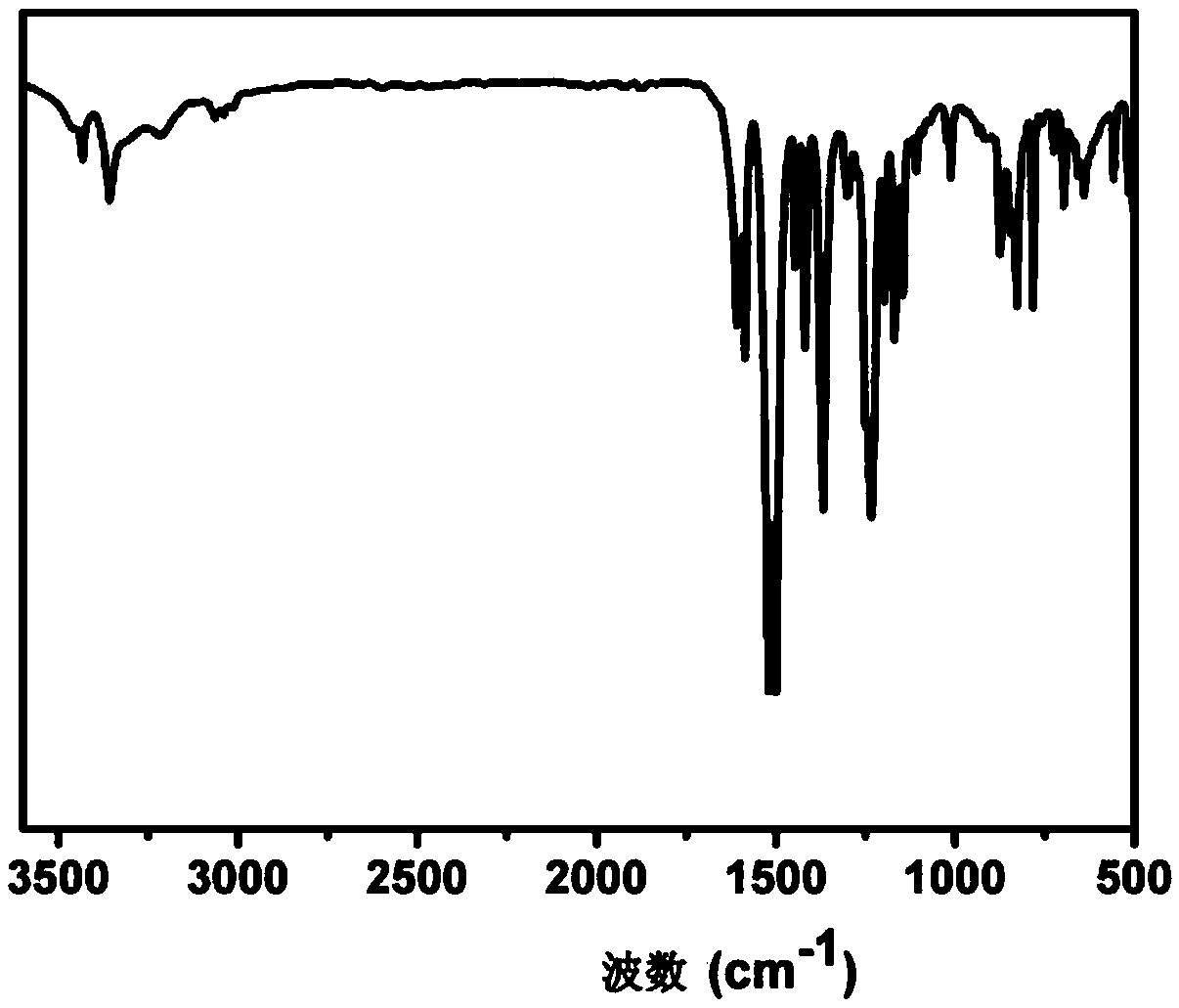
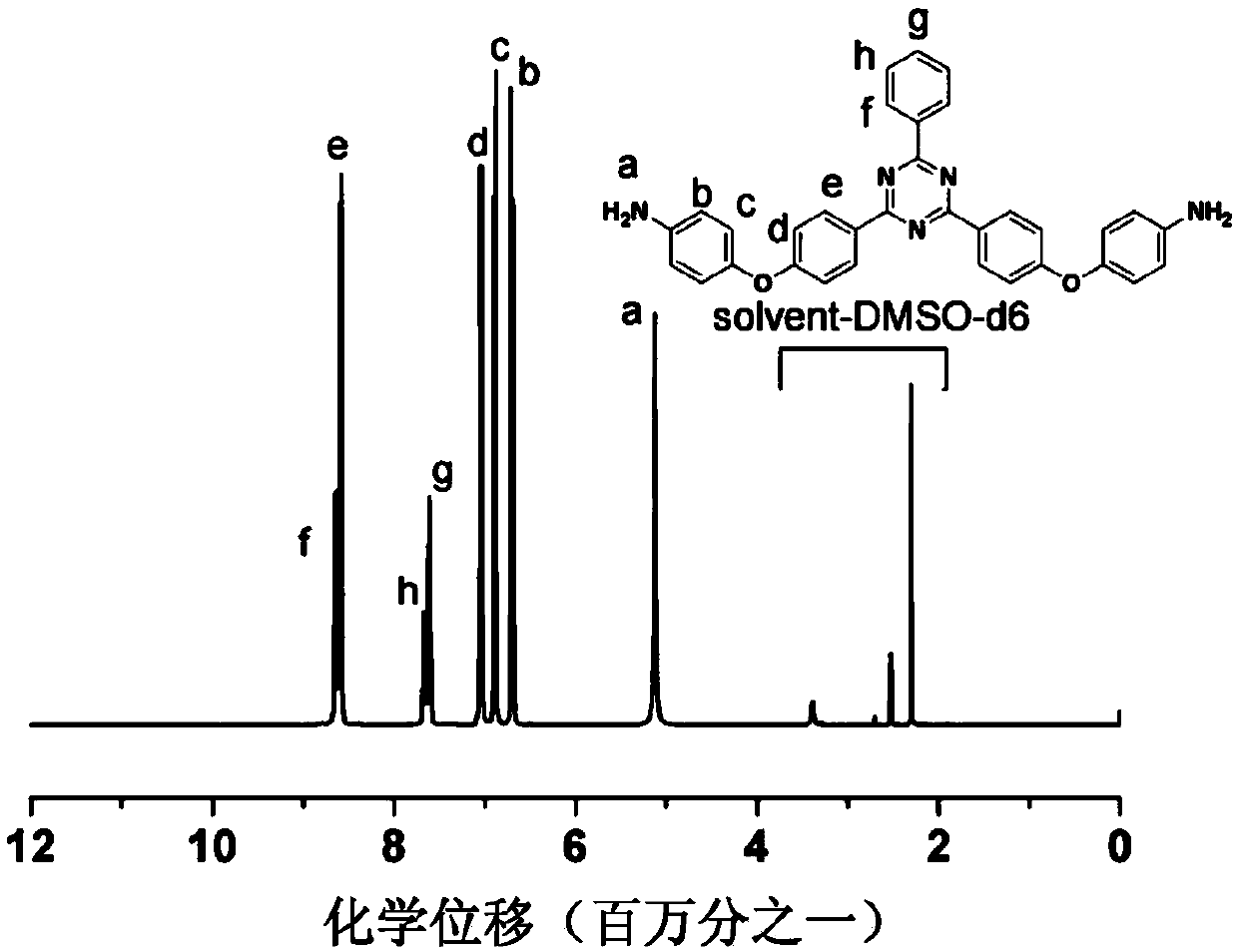
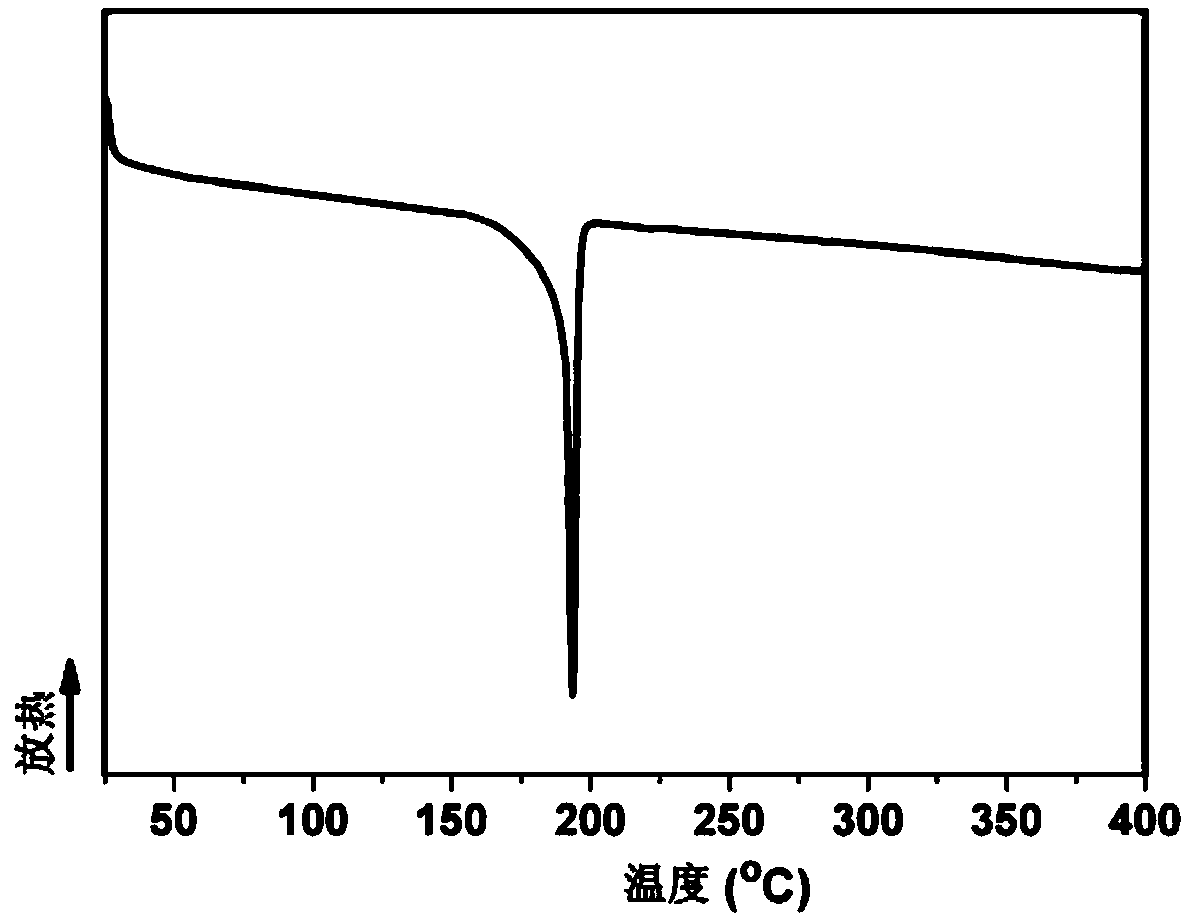
Aromatic secondary primary amine containing triaryl-s-triazine structure and ether bond and preparation method thereof
The invention discloses aromatic primary diamine containing a triaryl-s-triazine structure and an ether bond and a preparation method thereof, belonging to the technical field of synthesis of compounds. The aromatic primary diamine containing the triaryl-s-triazine structure and the ether bond has a structural formula as shown in a formula (I). The preparation method comprises the following steps: subjecting a dihalogen compound containing triaryl-s-triazine and shown in a formula (II) and a p-aminophenol compound as shown in a formula (III) to a nucleophilic substitution reaction; and then subjecting a reaction product to filtering, washing and drying so as to obtain the aromatic primary diamine containing the triaryl-s-triazine structure and the ether bond. The aromatic primary diamine containing the triaryl-s-triazine structure and the ether bond in the invention contains the triaryl-s-triazine structure with high rigidity, high thermal stability and strong polarity, so when the aromatic primary diamine is used as a monomer for synthesis of polyamide, polyimide and benzoxazine resin, the aromatic primary diamine can effectively improve the heat resistance and mechanical properties of a material and reduce the dielectric loss and water absorption rate of the material at the same time.
Owner:DALIAN UNIV OF TECH
A new method for preparing diarylmethyl substituted phosphonates
ActiveCN112010898BHigh selectivityHigh yieldGroup 5/15 element organic compoundsChemical recyclingPhosphorous acidPtru catalyst
The invention provides a method for synthesizing diarylmethylphosphonate derivatives containing different substituted functional groups with high efficiency and high selectivity. The method adopts silver tetrafluoroborate as a catalyst, and uses trialkyl phosphite and 4- Arylmethylene-2,6-di-tert-butyl-2,5-cyclohexadiene-1-ketone compounds are used as reaction substrates, and an organic solvent is added to the reaction system. The advantages of this method are as follows: the catalyst is cheap and easy to obtain; the substrate has high applicability; the reaction conditions are mild, safe and reliable; the selectivity of the obtained target product is close to 100%, and the yield is as high as 90%. The method solves the shortcomings of traditional synthesis of diarylmethyl substituted phosphonate derivatives, such as poor reaction selectivity, cumbersome reaction steps, low yield and the need to use environmentally harmful reagents, and has good industrial application prospects. The present invention also provides corresponding diarylmethyl substituted phosphonate derivatives with different substituted functional groups.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
A method for preparing organic phosphate compounds with p(o)-oh compound and aromatic hydrocarbon containing methyl substitution
ActiveCN107602609BHigh selectivityEfficient synthesisGroup 5/15 element organic compoundsPhosphoric Acid EstersPtru catalyst
The invention provides a high-efficiency and high-selectivity synthetic method for organic phosphate derivatives containing different substituted functional groups. The method uses tetrabutylammoniumiodide as a catalyst and uses a P(O)-OH-containing compound and a methyl-containing aromatic hydrocarbon compound as reaction substrates, and an organic solvent and an oxidant are added in the reaction system. The method has the advantages that the catalyst is cheap and easy to obtain, the substrate applicability is high, the reaction conditions are mild, safe and reliable, the selectivity of an obtained target product is close to 100%, and the yield is up to 90% or more. The method solves shortages in traditional organic phosphate ester compound synthetic methods that reaction selectivity ispoor, reaction steps are complicated, the yield is low, environmentally-harmful agents are used and the like, and has better industrial application prospects. The invention also provides the corresponding organic phosphate derivatives containing the different substituted functional groups.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
A method for preparing 2-iodo-1-phosphoryl substituted alkanes by efficient difunctionalization of alkenes
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
Method for preparing organic lactone
InactiveCN102702152BImprove stabilityEasy to makeOrganic chemistryMolecular sieve catalystsOrganic solventKetone
The invention provides a method for preparing organic lactone. The method is characterized in that: a load type catalyst Co-MFI having a hexagonal prism morphology is used as a catalyst, and the catalyst is prepared by a method of hydrothermal crystallization synthesis; organic ketone is used as a substrate, oxygen or air is used as oxygen sources, organic aldehyde substances are used as reducing agents, and an organic solvent is added into a reaction system. The method has the advantages that: the catalyst is easy to prepare; the reaction conditions are mild, safe, reliable, green and environment-friendly; the selectivity and the yield of target products approach to 100 percent; and the catalyst can be repeatedly recycled. According to the method, the problems of low safety, low yield and the like in the conventional preparation process of the lactone are solved, and the method has a good industrial application prospect.
Owner:HUNAN UNIV
Novel method for preparing phosphoryl azo compounds
ActiveCN112028933AThe reaction process is mild and easy to controlHigh yieldGroup 5/15 element organic compoundsArylTetrafluoroborate
The invention provides a method for preparing phosphoryl azo compounds with high efficiency and high selectivity; pyridine is used as alkali, an aryl diazonium tetrafluoroborate compound and a P(O)-Hcompound are used as reaction substrates, and an organic solvent is added into a reaction system. The method has the advantages that a catalyst is cheap and easily available; the substrate applicability is high; reaction conditions are mild, safe and reliable; the selectivity of the obtained target products is close to 100%, and the yield is high. The method overcomes the defects of harsh reactionconditions, poor reaction selectivity, complex experimental steps, low yield, need of reagents harmful to the environment and the like in a traditional synthesis of phosphoryl azo compounds, and hasfavorable industrial application prospects. The invention also provides the corresponding phosphoryl azo compounds containing different substituted functional groups.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
Method for preparing organic lactone
InactiveCN102603447BImprove stabilityEasy to makeCarboxylic acid ester formation/introductionMetal/metal-oxides/metal-hydroxide catalystsOrganic solventCobalt(II,III) oxide
The invention provides a method for preparing organic lactone. The method is characterized in that: cubic cobaltosic oxide is taken as a catalyst, and the catalyst is prepared with a hydrothermal method; and organic ketone is taken as a substrate, oxygen or air is taken as an oxygen source, an organic lactone substance is taken as a reducing agent, and an organic solvent is added into a reaction system. The method has the advantages that: the catalyst is easy to prepare; reaction conditions are mild, and the method is safe, reliable and environmentally-friendly; the selectivity and yield of an obtained target product are close to 100 percent; and the catalyst can be used for repeatedly. Due to the adoption of the method, the problems of poor safety, low yield and the like existing in the conventional lactone preparation process are solved, and the method has a good industrial application prospect.
Owner:HUNAN UNIV
A method for preparing o-diaryl phosphoryl substituted phenol derivatives
The invention provides a method for efficiently and highly selectively synthesizing o-diaryl phosphoryl-substituted phenolic derivatives containing different functional groups, which uses water as a catalyst to synthesize diaryl derivatives containing P(O)-H bonds The phosphorus oxy compound and the 4,4-dimethoxy-2,5-cyclohexadiene-1-ketone compound are used as reaction substrates, and an organic solvent is added to the reaction system. The method has the advantages that the catalyst is cheap and easy to obtain; the substrate has high applicability; the reaction condition is mild, safe and reliable; the yield of the obtained target product is as high as more than 90%. The method solves the shortcomings of traditional synthesis of o-diaryl phosphoryl substituted phenol derivatives such as poor reaction selectivity, cumbersome reaction steps, low yield, and the need to use harmful reagents to the environment, and has good industrial application prospects. The present invention also provides corresponding o-diaryl phosphoryl substituted phenol derivatives containing different functional groups.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
A novel method for efficiently preparing diarylmethyl-substituted organic phosphonates with p(o)-h compounds
ActiveCN109456362BHigh selectivityHigh yieldGroup 5/15 element organic compoundsPtru catalystOrganosolv
The invention provides a method for efficiently and selectively synthesizing diaryl methyl substituted organic phosphonate derivatives containing different substitute functional groups. The method uses cesium carbonate as a catalyst, and uses a P (O)-H compound and 4-arylmethylene-2, 6-di-tert-butyl 2, 5-cyclohexadiene-1-ketone compounds as reactants; an organic solvent is added into a reaction system. The method has the advantages that the catalyst is cheap and easy to obtain; the reactants are high in applicability; the reaction is mild in conditions, safe and reliable; the selectivity of the obtained target product is close to 100%, and the yield is as high as 90% or above. The method solves the problems that the conventional synthetic diaryl methyl substituted organic phosphonate derivatives are poor in reaction selectivity, tedious in reaction steps and low in yield, needs to use environmentally harmful reagents, and the like; the method has a good industrial application prospect.The invention also provides the corresponding diaryl methyl substituted organic phosphonate derivatives containing the different substitute functional groups.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
A method for preparing organophosphate compounds by efficiently esterifying p(o)-oh compounds with phenol
ActiveCN107082789BEfficient synthesisHigh selectivityGroup 5/15 element organic compoundsPhosphoric Acid EstersOrganosolv
The invention provides a method for preparing an organophosphate compound through efficient esterification of a P(O)-OH-containing compound and phenol. The method realizes efficient and highly selective synthesis of organophosphate derivatives containing different substituted functional groups. A carbonyl diimidazole compound is used as a condensation reagent, a P(O)-OH-containing compound and phenol are used as reaction substrates, and an organic solvent is added into the reaction system. The catalyst is cheap and easy to acquire, the applicability to the substrate is high, the reaction conditions are mild, safe and reliable, the selectivity of the target product is close to 100% and the yield is 90% or more. The method solves the problem that the traditional method for synthesis of an organophosphate compound containing an aromatic functional group has poor reaction selectivity, complex reaction steps and a low yield, needs reagents harmful for the environment, and has good industrial application prospects. The invention also provides an organophosphate derivative containing different aromatic group substituents.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
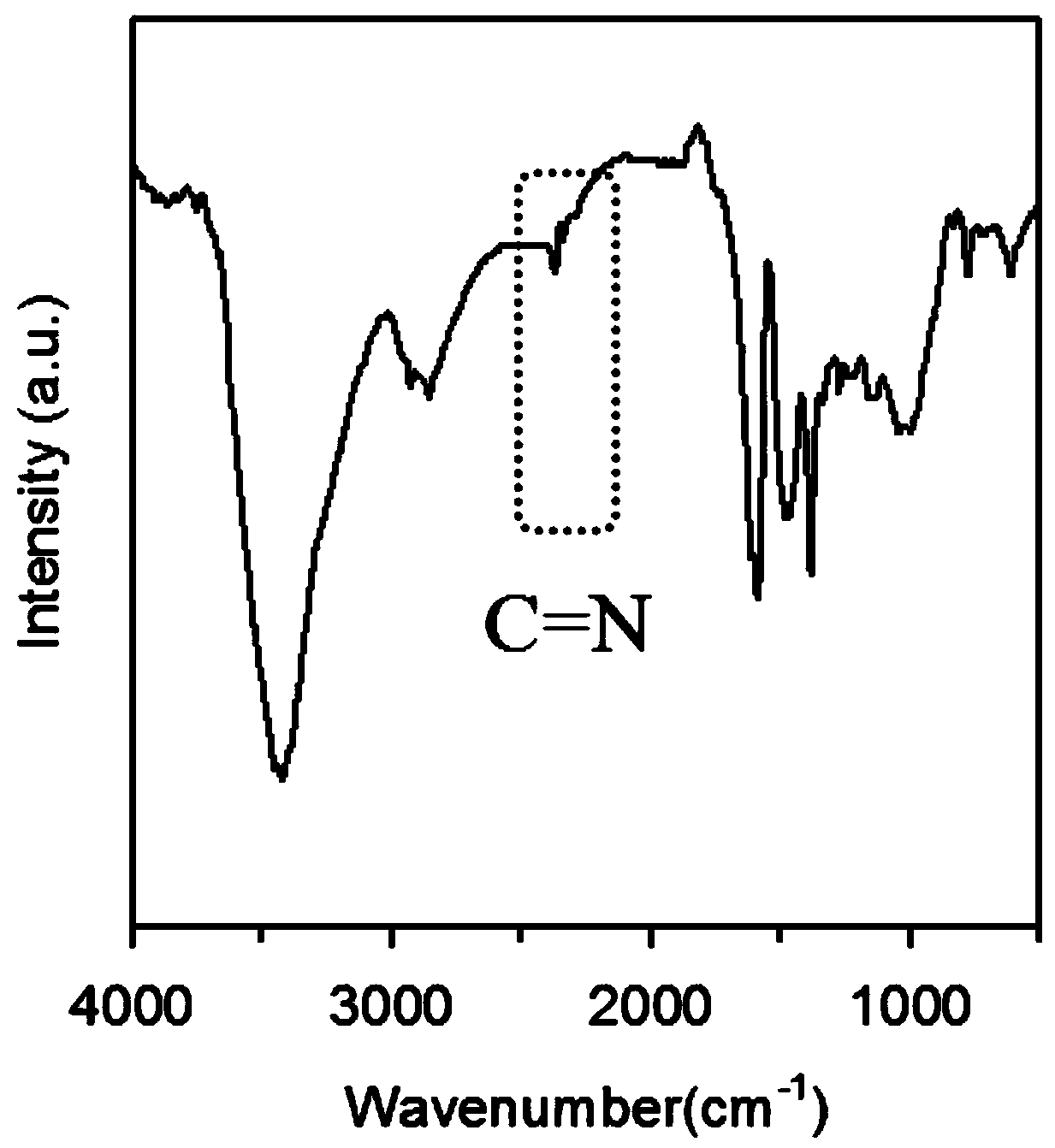
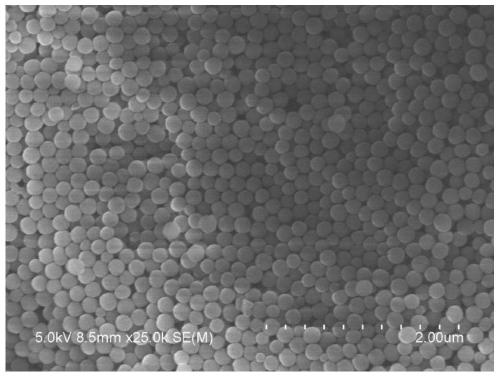
A kind of size-controllable Schiff base polymer nanoparticle and its preparation method
ActiveCN107099032BControllable particle size preparationEasy to separate and purifyEngineeringAminopyridines
The invention discloses a size-controllable Schiff base-type polymer nano-particle, which is prepared through a Schiff base reaction to 2,6-diaminopyridine and formalin, wherein molar ratio of the 2,6-diaminopyridine to formaldehyde in the formalin is 1:2-18. The invention also provides a preparation method of the size-controllable Schiff base-type polymer nano-particle with the 2,6-diaminopyridine and formalin as raw materials. The particle size of the size-controllable Schiff base-type polymer nano-particle can reach 120 nm to lowest. The preparation method is simple and environment-friendly and is carried out entirely in deionized water, has mild and easy-to-control reaction conditions, saves production cost and is easy to carry out in industrial production.
Owner:ANHUI UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

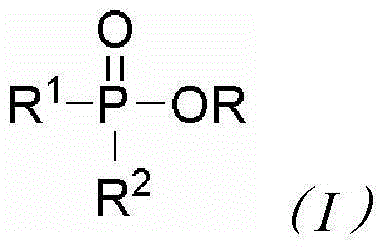


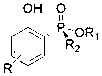
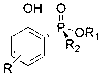

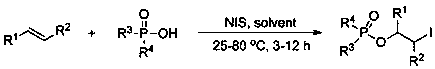
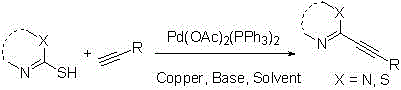
![A new method for preparing 2'-iodo[1,1'-biaryl]-2-organophosphonate compounds A new method for preparing 2'-iodo[1,1'-biaryl]-2-organophosphonate compounds](https://images-eureka.patsnap.com/patent_img/30104d7f-693a-41ac-bc22-ef6f3e31a00d/DEST_PATH_IMAGE001.png)