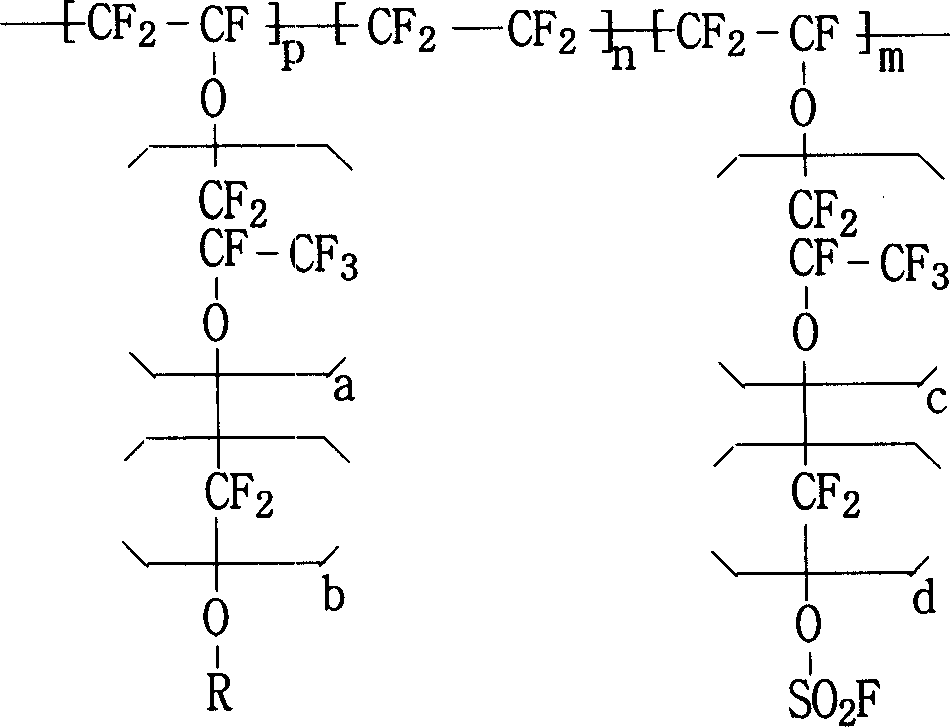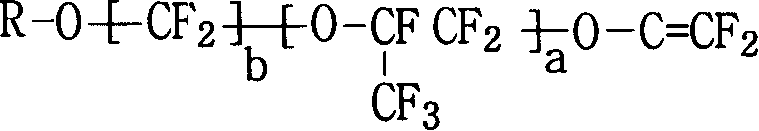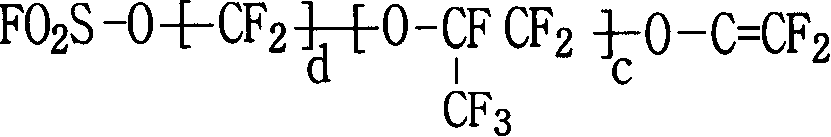Fluor resin with sulfuryl fluoride and aether terminal group lateral group, synthesizing method and application thereof
A technology of sulfonyl fluoride, ether, and sulfonyl fluoride end group alkenyl ether, which is applied in the field of ternary copolymerization perfluorosulfonic acid resin and its synthesis, and can solve the problems of heat resistance and chemical corrosion resistance reduction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment 1: (solution polymerization, fluorocarbon solvent, perfluoroalkyl acyl peroxide)
[0088] After cleaning and drying the reactor, it was vacuum-filled three times with nitrogen, vacuum-filled tetrafluoroethylene monomer to 0.08MPa, and then vacuum-filled to 0.0001MPa, and then 300g perfluoromethylcyclobutane, 100g perfluorosulfonyl mono Body (CF 2 =CFO-CF 2 CFCF 3 O-CF 2 CF 2 SO 2 F), 5g 6-methoxy-propyl vinyl ether (CF 2 =CFO-CF 2 CF 2 CF 2 -OCH 3 ) was added to the reaction kettle, the temperature was raised to 45°C, and 20ml containing 0.02g perfluorobutyryl compound (CF 3 CF 2 CF 2 CO-OO-CCF 2 CF 2 CF 3 ), into tetrafluoroethylene (CF 2 = CF 2 ) until the pressure reaches 1MPa, and maintain the pressure at 0.8-1.0MPa to continue the reaction. When the amount of tetrafluoroethylene added reaches 110g, stop adding and allow the reaction to continue. When the pressure in the still was reduced to 0.2MPa, the reaction was stopped and the unrea...
Embodiment 2
[0096] Embodiment 2: (solution polymerization, azo initiator)
[0097] After cleaning and drying the reactor, vacuumize and fill nitrogen three times, then vacuumize and fill tetrafluoroethylene monomer to 0.08MPa, and then vacuumize to 0.0001MPa, then add 300g perfluoromethylcyclobutane, 100g perfluorosulfonyl Monomer (CF 2 =CFO-CF 2 CFCF 3 O-CF 2 CF 2 SO 2 F), 5g 6-methoxy-propyl vinyl ether (CF 2 =CFO-CF 2 CF 2 CF 2 -OCH 3 ), 0.2g of azobisisobutyronitrile were added to the reaction kettle, the temperature was raised to 60°C, and 20ml of perfluorobutyryl compound containing peroxide was added with a metering pump, and tetrafluoroethylene (CF 2 = CF 2 ) until the pressure reaches 1MPa, and maintain the pressure at 0.8-1.0MPa to continue the reaction. When the amount of tetrafluoroethylene added reaches 110g, stop adding and allow the reaction to continue. When the pressure in the still was reduced to 0.2MPa, the reaction was stopped and the unreacted tetrafluoroe...
Embodiment 3
[0098] Embodiment 3: (dispersion polymerization, in water, persulfate is made initiator)
[0099] After the reactor was cleaned, add 3g of perfluoroalkoxyammonium carboxylate (CF 3 CF 3 CF 2 OCFCF 3 CF 2 -OCFCF 3 COONH 4 ), 500g of pure water, evacuated and filled with nitrogen three times, then evacuated and filled with tetrafluoroethylene monomer to 0.08MPa, and then evacuated to 0.0001MPa, 100g of perfluorosulfonyl monomer (CF 2 =CFO-CF 2 CFCF 3 O-CF 2 CF 2 SO 2 F), 8g 6-methoxy-propyl vinyl ether (CF 2 =CFO-CF 2 CF 2 CF 2 -OCH 3 ) into the reaction kettle, the temperature was raised to 80°C, and 20ml of aqueous solution containing 0.05g of potassium persulfate was added with a metering pump, and tetrafluoroethylene (CF 2 = CF 2 )80g, the reaction begins. When the pressure in the kettle was reduced to 0.1MPa, the reaction was stopped, and the unreacted tetrafluoroethylene monomer was recovered. Release the material, and unreacted monomers will precipitate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com