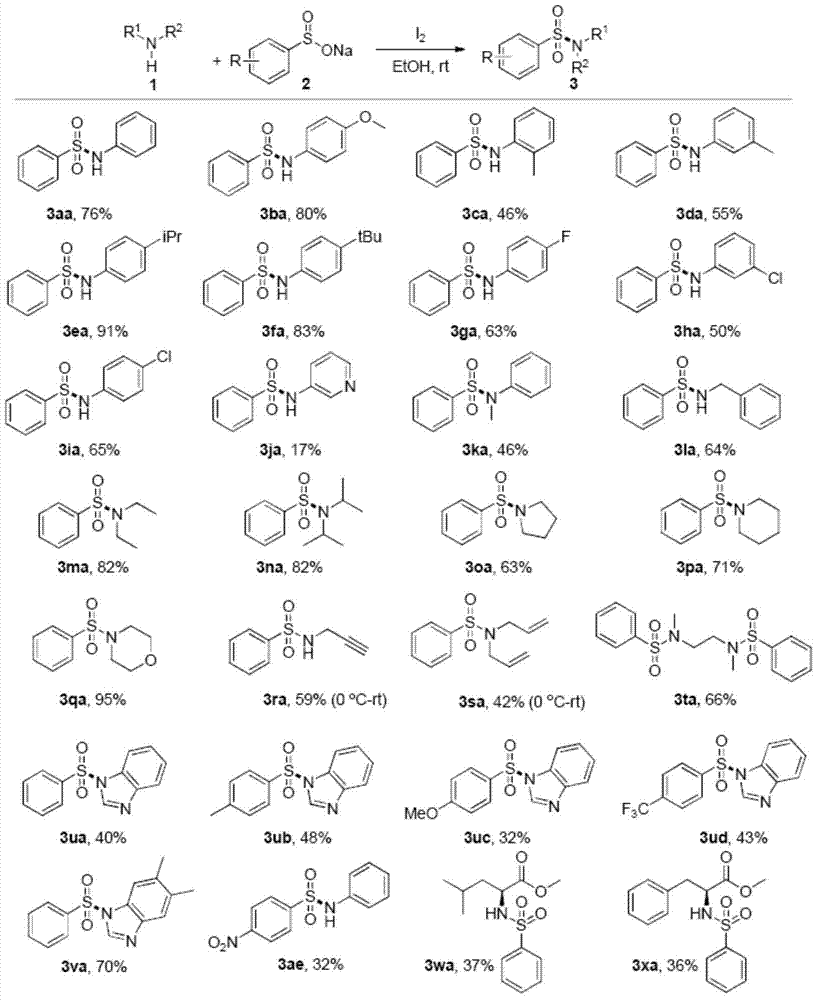
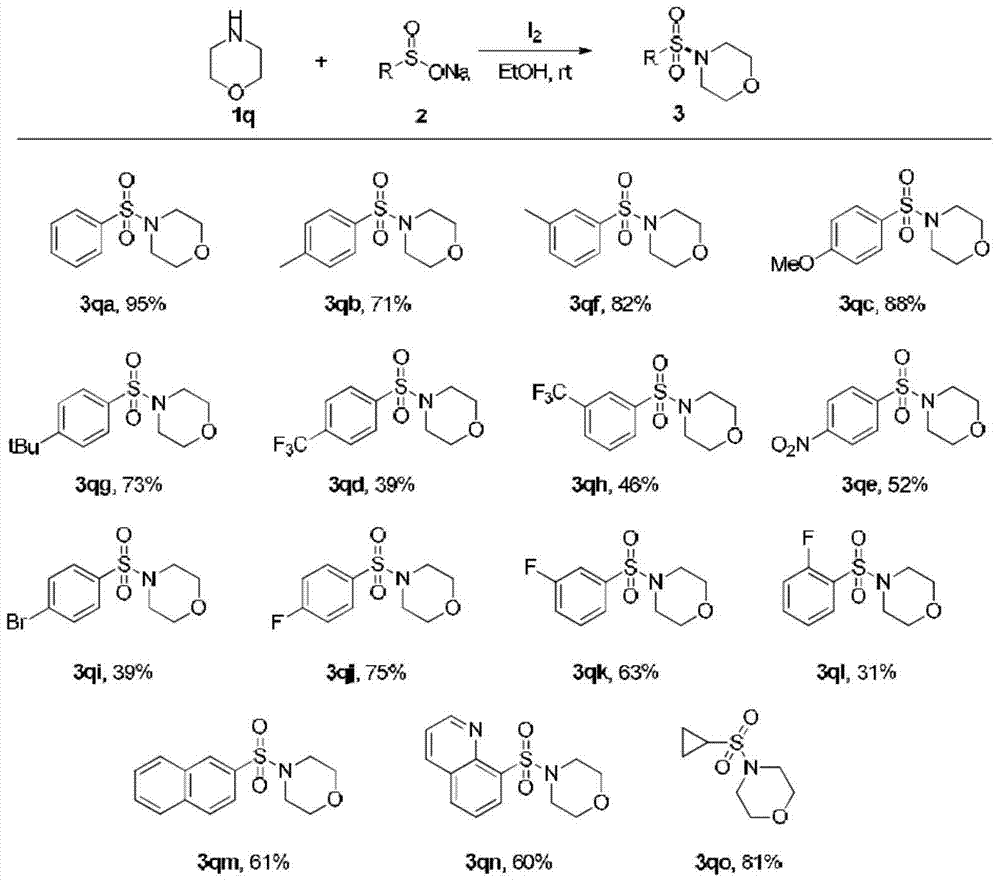
Method for preparing sulfonamide and derivatives thereof
A technology of derivatives and sulfonamides, which is applied in the field of preparation of sulfonamides and their derivatives, can solve the problems of poor tolerance of functional groups, harsh reaction conditions, and contamination of transition metals, and achieve mild reaction conditions, simple operation, and easy purchase Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Add sodium benzenesulfinate (1mmol) and I in a round bottom flask 2 (0.5mmol), the mixed solution was stirred at room temperature for 20 minutes, then acetonitrile (2ml) and aniline (0.5mmol) were added, and the mixed solution was stirred at room temperature for 18h.
[0026] (2) After the reaction was completed, 10% concentration of sodium thiosulfate (20ml) was added. Ethyl acetate (20 mL*3) was added to the reaction solution to extract the aqueous phase, and the organic phase was dried over anhydrous sodium sulfate.
[0027] (3) The organic phase was spin-dried by a rotary evaporator, and the product was purified by a silica gel column. The isolated yield of the product N-phenylbenzenesulfonamide was 60%.
Embodiment 2
[0029] (1) Add sodium benzenesulfinate (1mmol) and I in a round bottom flask 2 (0.5mmol), the mixed solution was stirred at room temperature for 20 minutes, then dichloromethane (2ml) and aniline (0.5mmol) were added, and the mixed solution was stirred at room temperature for 18h.
[0030] Steps (2) and (3) are the same as in Example 1, and the isolated yield of the product N-phenylbenzenesulfonamide is 69%.
Embodiment 3
[0032] (1) Add sodium benzenesulfinate (1mmol) and I in a round bottom flask 2 (0.5mmol), the mixed solution was stirred at room temperature for 20 minutes, then water (2ml) and aniline (0.5mmol) were added, and the mixed solution was stirred at room temperature for 18h.
[0033] Steps (2) and (3) are the same as in Example 1, and the isolated yield of the product N-phenylbenzenesulfonamide is 73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com