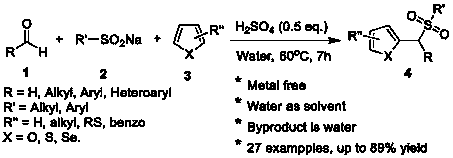
Synthesis method of aryl(chalcogen-heteroaryl)methyl sulfone
A synthetic method and heteroaryl technology, which is applied in the field of synthesis of aryl (chalcogenoheteroaryl) methyl sulfones, and can solve problems that have not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
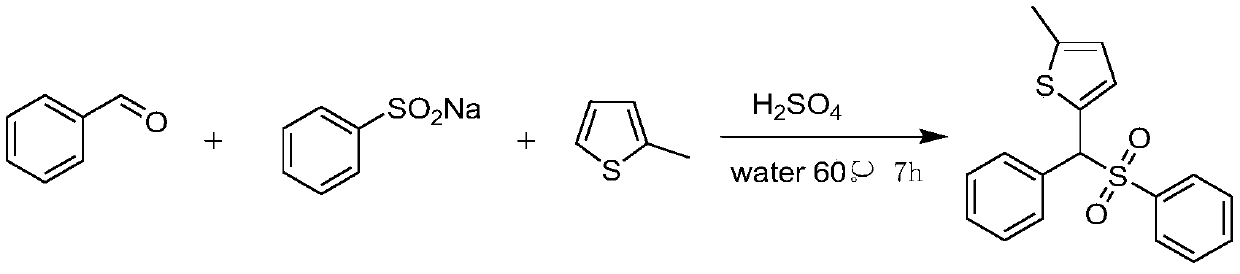
[0011] Benzaldehyde (1.5 mmol), 2-methylthiophene (1 mmol), sulfuric acid (0.5 equiv) and 2 mL of water were added to a 25 mL glass test tube equipped with a stir bar. The tube was stirred for 15 minutes in a preheated oil bath at 60°C, then sodium benzenesulfinate (1 mmol) was added slowly. The reaction mixture was stirred at 60 °C. After 7 hours, the progress of the reaction was checked by TLC and the reaction was confirmed to be complete. The reaction mixture was cooled to room temperature. Water (10 mL) was then added to the reaction mixture, which was extracted three times with ethyl acetate (10 mL). The combined organic layers were washed with anhydrous MgSO 4 Dry, filter, and concentrate under reduced pressure. The residue was purified by flash column chromatography on silica gel (petroleum ether:ethyl acetate=10:1 as eluent), after purification, 2-methyl-5-(phenyl(benzenesulfonyl)methyl) was obtained as white solid Thiophene yield was 82%. The reaction equation i...
Embodiment 2
[0019] 4-Fluorobenzaldehyde was used instead of benzaldehyde in Example 1 to obtain a white solid 2-((4-fluorophenyl)(benzenesulfonyl)methyl)-5-methylthiophene with a yield of 89%.
[0020] 1 H NMR (400MHz, Chloroform-d) δ7.76–7.33(m,7H),7.12–6.87(m,3H),6.63(dq,J=3.5, 1.1Hz,1H),5.44(s,1H), 2.45(s,3H).
[0021] 13 C NMR (101MHz, cdcl 3 )δ164.23, 161.75, 142.17, 137.30, 133.68, 131.82, 131.74, 130.71, 129.78, 129.09, 128.69, 128.49, 125.15, 115.76, 115.55, 71.64, 15.29.
[0022] HRMS (ESI): calculated for C 18 h 15 FO 2 S 2 Na[M+Na] + =369.0395,foundC 18 h 15 FO 2 S 2 Na[M+ Na] + =369.0373.
[0023] Melting point: 129-130℃.
Embodiment 3
[0025] 2-Chlorobenzaldehyde was used instead of benzaldehyde in Example 1 to obtain a white solid 2-((2-chlorophenyl)(benzenesulfonyl)methyl)-5-methylthiophene with a yield of 88%.
[0026] 1 H NMR (400MHz, Chloroform-d) δ8.23–8.16(m,1H),7.74–7.65(m,2H),7.63–7.55(m,1H),7.47–7.35(m,3H),7.26–7.23 (m,2H),7.00(d,J=3.5Hz,1H),6.66(dd,J=3.6,1.2Hz,1H),6.29(s,1H),2.47(d,J=1.1Hz,3H) .
[0027] 13 C NMR (101MHz, CDCl 3 )δ142.43, 137.63, 134.73, 133.77, 130.99, 130.70, 130.26, 130.13, 130.10, 129.55, 129.08, 128.70, 127.23, 125.15, 66.83, 15.36.
[0028] HRMS (ESI): calculated for C 18 h 15 ClO 2 S 2 Na[M+Na] + =385.0100,foundC 18 h 15 ClO 2 S 2 Na[M +Na] + =385.0089.
[0029] Melting point: 100-101℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com