Chiral sulfonamide derivative and preparation method and application thereof
A technology for sulfonamides and derivatives, applied in the field of chiral sulfonamide derivatives and their preparation, can solve the problems of unfavorable sulfonamide derivatives using industrial synthesis, non-recyclable chemical waste, low atom economy and the like , to achieve the effect of low cost, less waste and fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
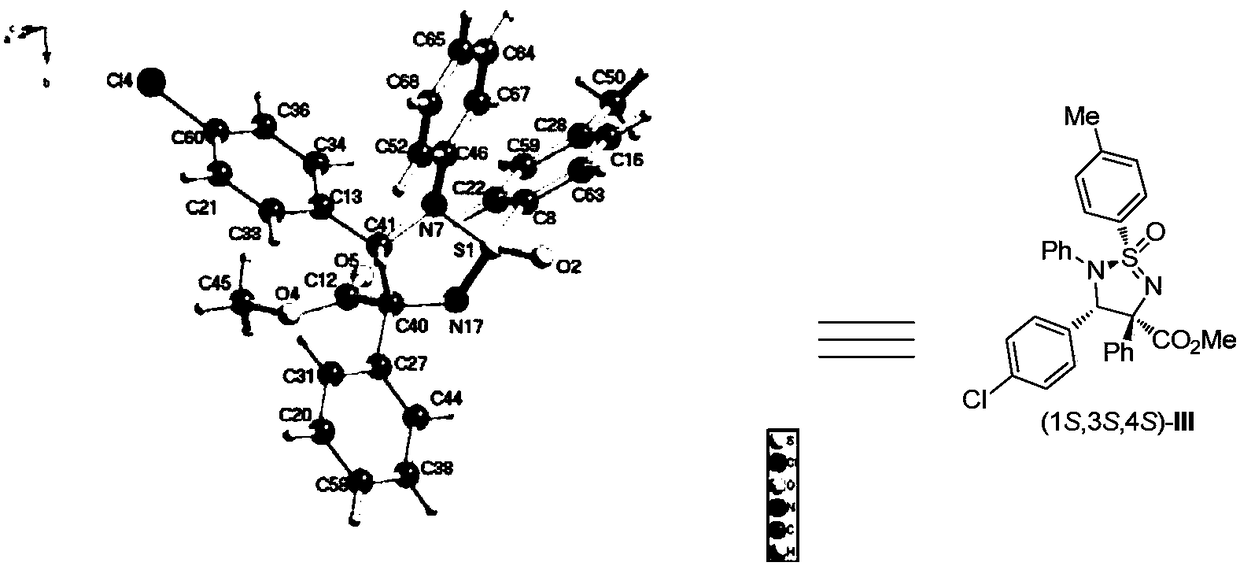
[0088] The present invention synthesizes the preparation method of chiral sulfonamide derivatives, sulfonamide, imine, rhodium acetate, Molecular sieves are dissolved in an organic solvent to prepare a mixed solution; aryl diazoacetate is dissolved in an organic solvent to prepare a diazo compound solution; at -10°C, the diazo compound solution is added to the aforementioned mixed solution with a syringe pump; at the same time Stir vigorously; after the diazo compound solution is added dropwise, continue to stir for 60 minutes at -10°C until the diazo compound is completely consumed; the crude product is subjected to column chromatography (using ethyl acetate:petroleum ether=1:20~1: 10 is the eluent) to obtain pure product.
[0089] The synthesis reaction process is as follows:
[0090]
[0091] In reaction formula (II),
[0092] R is aryl or alkyl, selected from phenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 4-methoxyphenyl, 3,4-dimethoxy phenyl, 2-thienyl, 2-...
Embodiment 1
[0097]
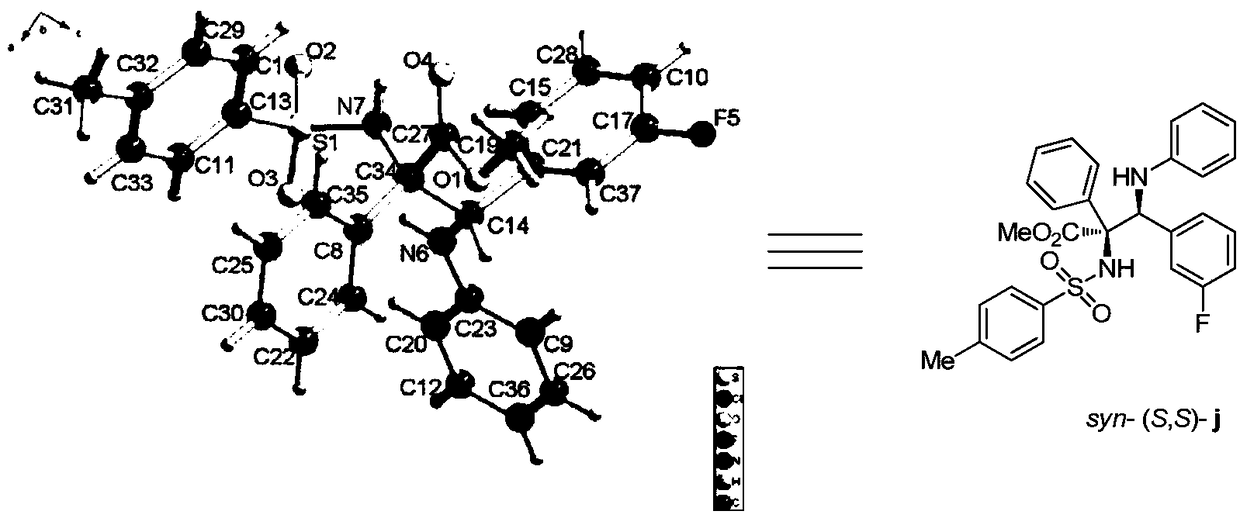
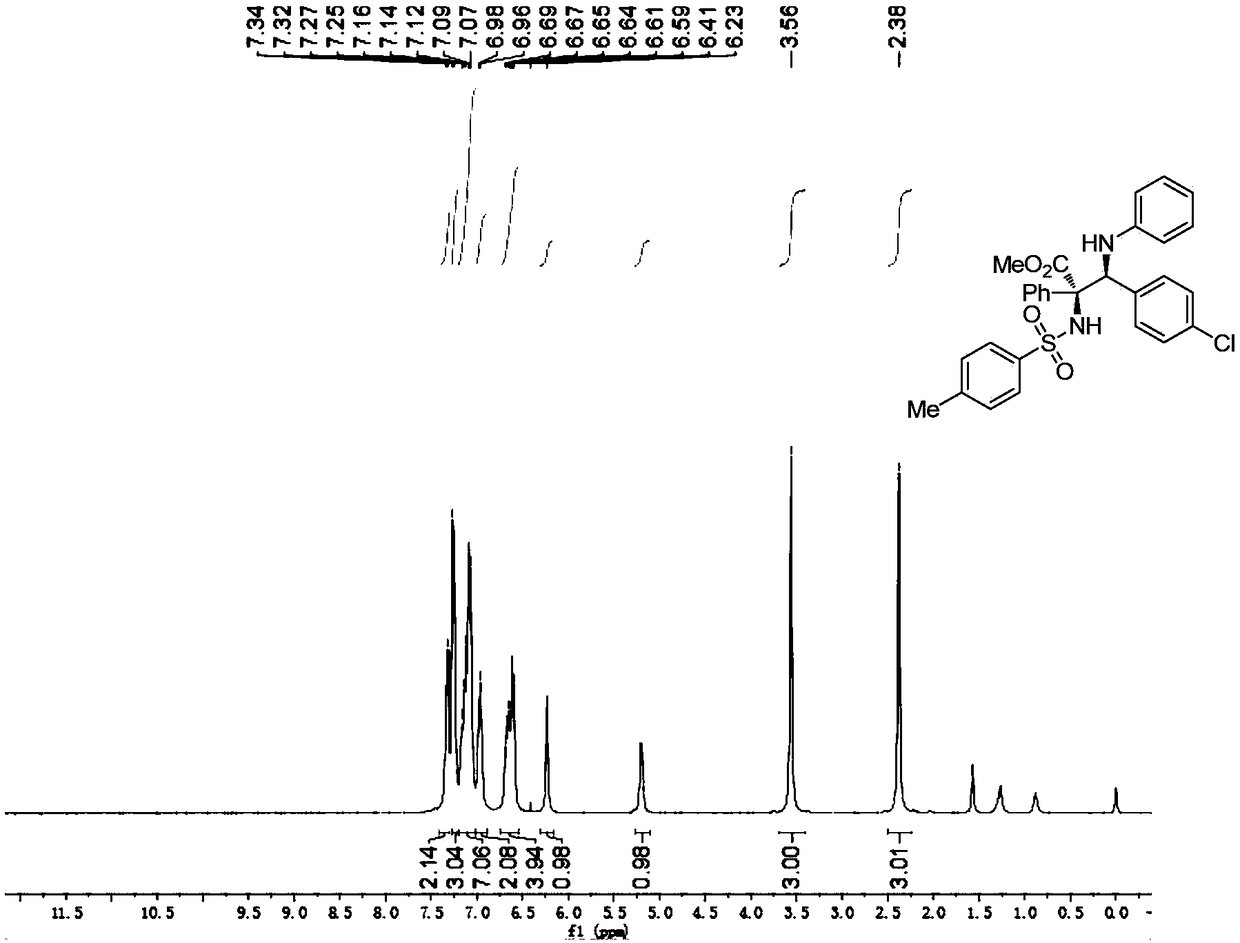
[0098] P-toluenesulfonamide (0.36mmol), p-chlorobenzylidene aniline (0.30mmol), rhodium acetate (0.006mmol), (R)-3,3'-bis(triphenylsilyl)binaphthol phosphate (0.03mmol), Molecular sieves (150 mg) were dissolved in 3.0 mL of anhydrous toluene to prepare mixed solution A; methyl phenyldiazoacetate was dissolved in 1.0 mL of anhydrous toluene to prepare diazo compound solution B; at -10 ° C, solution B At -10°C, the mixed solution A was added with a syringe pump within 1 hour. After injecting solution B, the reaction system was stirred at -10°C for 60 minutes. After the reaction was completed, the filtrate was filtered and the solvent was removed by rotary evaporation, and then the crude product was purified by column chromatography to obtain a pure product as a white solid. Its structure is shown in formula (a). The isolated yield of the product was 83%, the dr value was greater than 20:1, and the ee value was 99%. of the product 1 H NMR schematic as image 3 A...
Embodiment 2
[0101]
[0102] P-4-tert-butylbenzenesulfonamide (0.36mmol), p-chlorobenzylidene aniline (0.30mmol), rhodium acetate (0.006mmol), (R)-3,3'-bis(triphenylsilyl) Binaphthol phosphate (0.03mmol), Molecular sieves (150 mg) were dissolved in 3.0 mL of anhydrous toluene to prepare mixed solution A; methyl phenyldiazoacetate was dissolved in 1.0 mL of anhydrous toluene to prepare diazo compound solution B; at -10 ° C, solution B At -10°C, the mixed solution A was added with a syringe pump within 1 hour. After injecting solution B, the reaction system was stirred at -10°C for 60 minutes. After the reaction was completed, the filtrate was filtered and the solvent was removed by rotary evaporation, and then the crude product was purified by column chromatography to obtain a pure product as a white solid. Its structure is shown in formula (b). The isolated yield of the product was 78%, the dr value was greater than 20:1, and the ee value was 90%. of the product 1 H NMR schematic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com