Synthesizing method of methyl phenyl sulfoxide
A technology of methyl sulfoxide and synthesis methods, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of restricting wide application, low efficiency, increasing costs, etc., and achieve simple and reasonable process conditions, The effect of rapid reaction speed and good conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add (10ml) acetonitrile and 885mg (2.50mmol) F-TEDA-BFI solution to a 25ml anhydrous and oxygen-free bottle, and add dropwise phenylmethyl sulfide (2.0 mmol), then stirred at room temperature for 10 min, then added 347 uL (2.5 mmol) of triethylamine, and continued to stir for 10 min. After the reaction was over, the reaction solution was poured into water, and 15ml of dichloromethane was added for extraction, then with Na 2 CO 3 The solution was dried and filtered to obtain methyl phenyl sulfoxide with a yield of 54%.
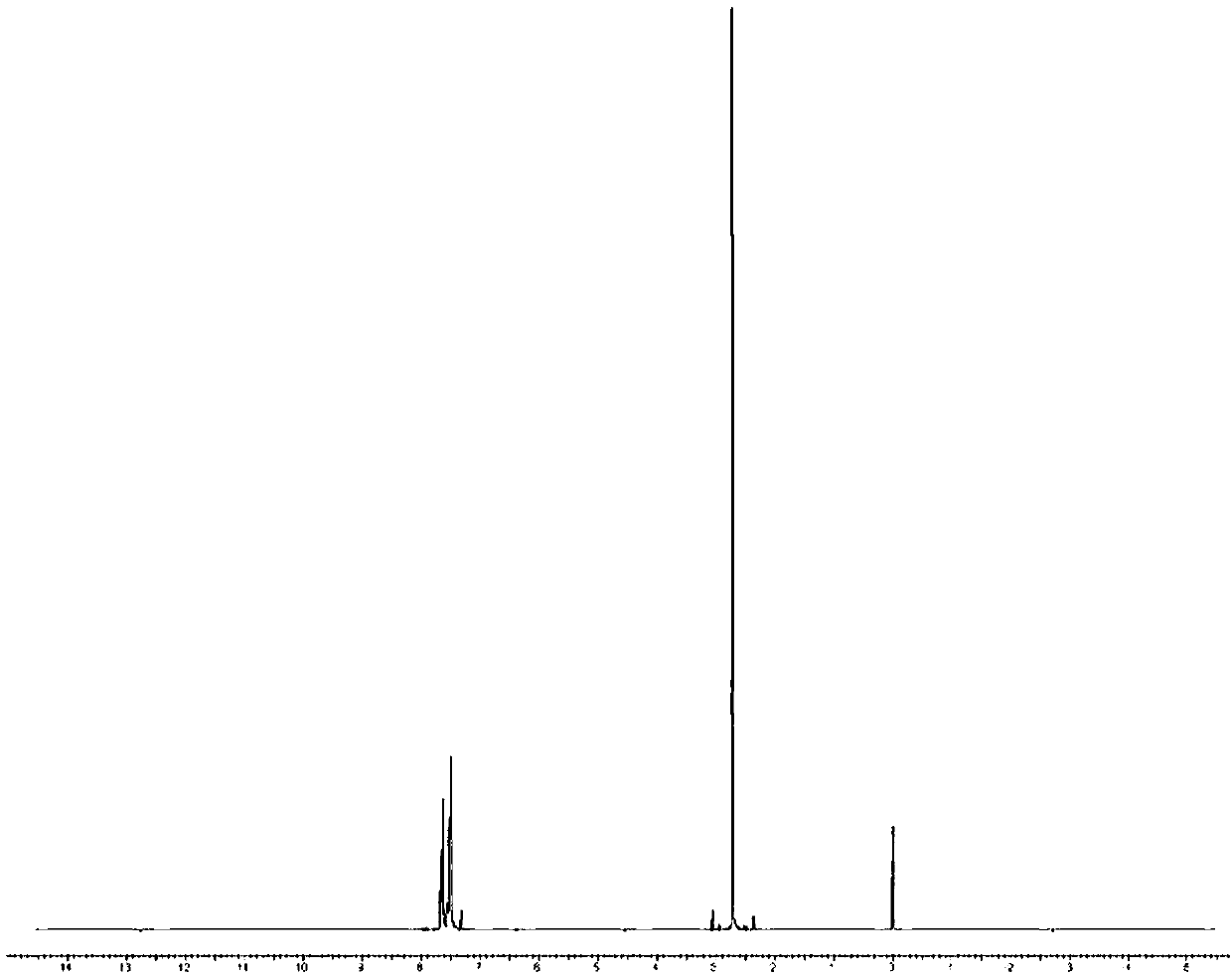
[0032] NMR 1H(CDCl3,300MHz):δ2.72(s,3H),7.49-7.55(3H),7.63-7.66(2H)
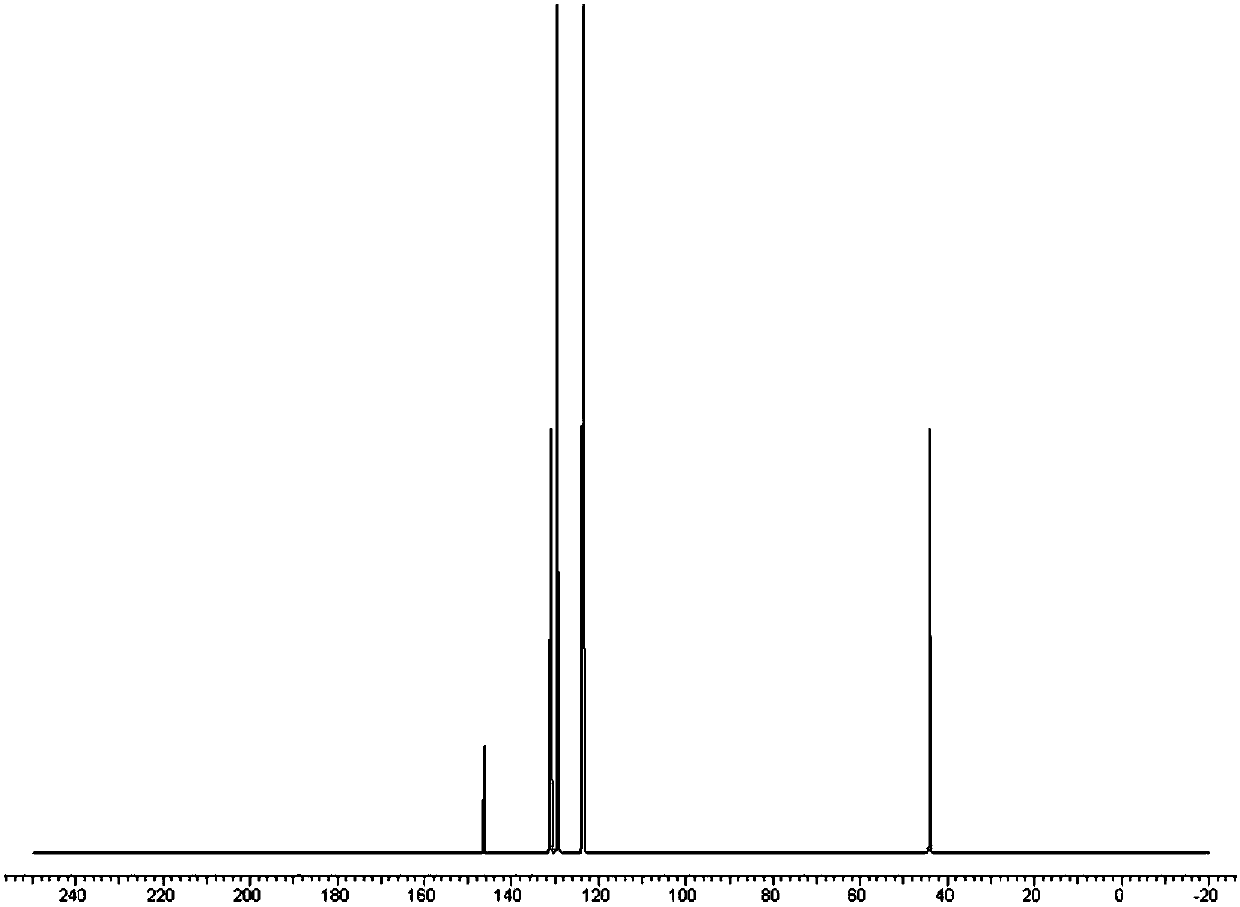
[0033] NMR 13C (CDCl3, 100MHz): δ145.4(Cq), 130.8(CH), 129.2(CH), 123.3(CH), 43.7(CH3)
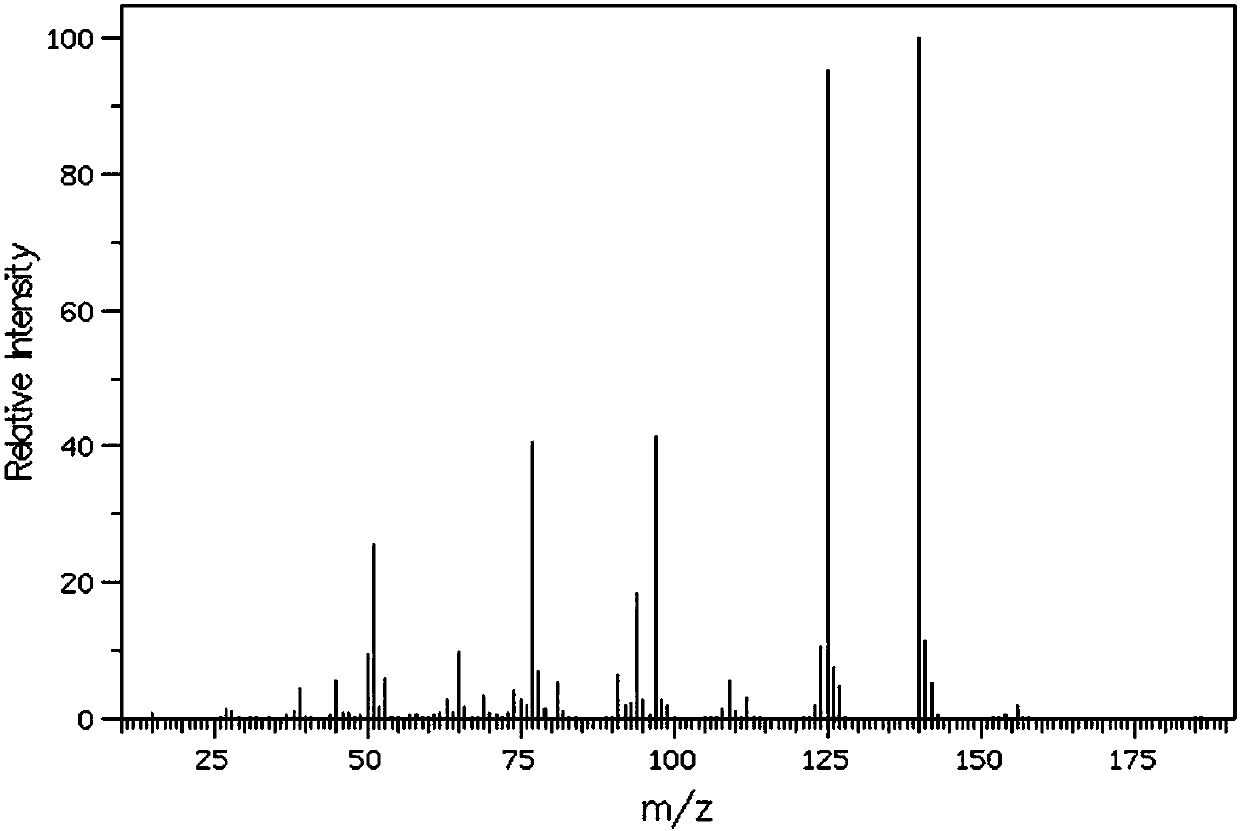
[0034] GCMS(EI)Calcd.for C 7 h 8 OS 140.20, found 140.04.
Embodiment 2
[0036] Add (12ml) acetonitrile and 885mg (2.50mmol) F-TEDA-BFI solution to a 25ml anhydrous and oxygen-free bottle, and add dropwise methyl p-toluene sulfide (2.0 mmol), then stirred at room temperature for 10 min, then added 347 uL (2.5 mmol) of triethylamine, and continued to stir for 10 min. After the reaction was over, the reaction solution was poured into water, and 15ml of dichloromethane was added for extraction, then with Na 2 CO 3 The solution was dried and filtered to obtain methyl p-tolylsulfoxide with a yield of 61%.
[0037] NMR 1H(CDCl3,400MHz):δ2.41(s,3H),2.70(s,3H),7.32(d,J=8.1Hz,2H),7.53(d,J=8.1Hz,2H)
[0038] NMR 13C (CDCl3, 100MHz): δ142.3(Cq), 141.5(Cq), 130.0(CH), 123.5(CH), 43.8(CH3), 21.3(CH3)
[0039] GCMS(EI)Calcd.for C 8 h 10 OS 154.23, found 154.05.
Embodiment 3
[0041] Add (10ml) acetonitrile and 886mg (2.50mmol) F-TEDA-BFI solution to a 25ml anhydrous and oxygen-free bottle, and add 4-chlorophenylmethylsulfur pre-dissolved in 1.0ml acetonitrile dropwise under a nitrogen atmosphere ether (ie thioanisole) (2.0 mmol), then stirred at room temperature for 10 min, then added 350 uL (2.5 mmol) of triethylamine, and continued to stir for 10 min. After the reaction was over, the reaction solution was poured into water, and 15ml of dichloromethane was added for extraction, then with Na 2 CO 3 The solution was dried and filtered to obtain p-chlorophenylmethyl sulfoxide with a yield of 68%.
[0042] 1HNMR (500MHz, CDCl3) δ (ppm): 7.63-7.60 (m, 2H), 7.55-7.52 (m, 2H), 2.74 (s, 3H);
[0043] 13C NMR (126MHz, CDCl3) δ (ppm): 144.2, 137.3, 131.1, 129.7, 128.9, 125.0, 44.0.
[0044] GCMS(EI)Calcd.for C 7 h 7 ClOS 174.64, found 173.99.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com