Novel synthesis method of gem-difluoroalkane and alpha-fluorocarboxylic acid
A technology for fluorinated carboxylic acid and difluoroalkane, which is applied in the field of novel synthesis of geminal difluoroalkane and α-fluorinated carboxylic acid, and can solve the problem that starting materials are difficult to obtain substrate range, poor functional group compatibility, harsh reaction conditions, etc. problems, to achieve good chemical selectivity and functional group compatibility, mild conditions, and simple raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
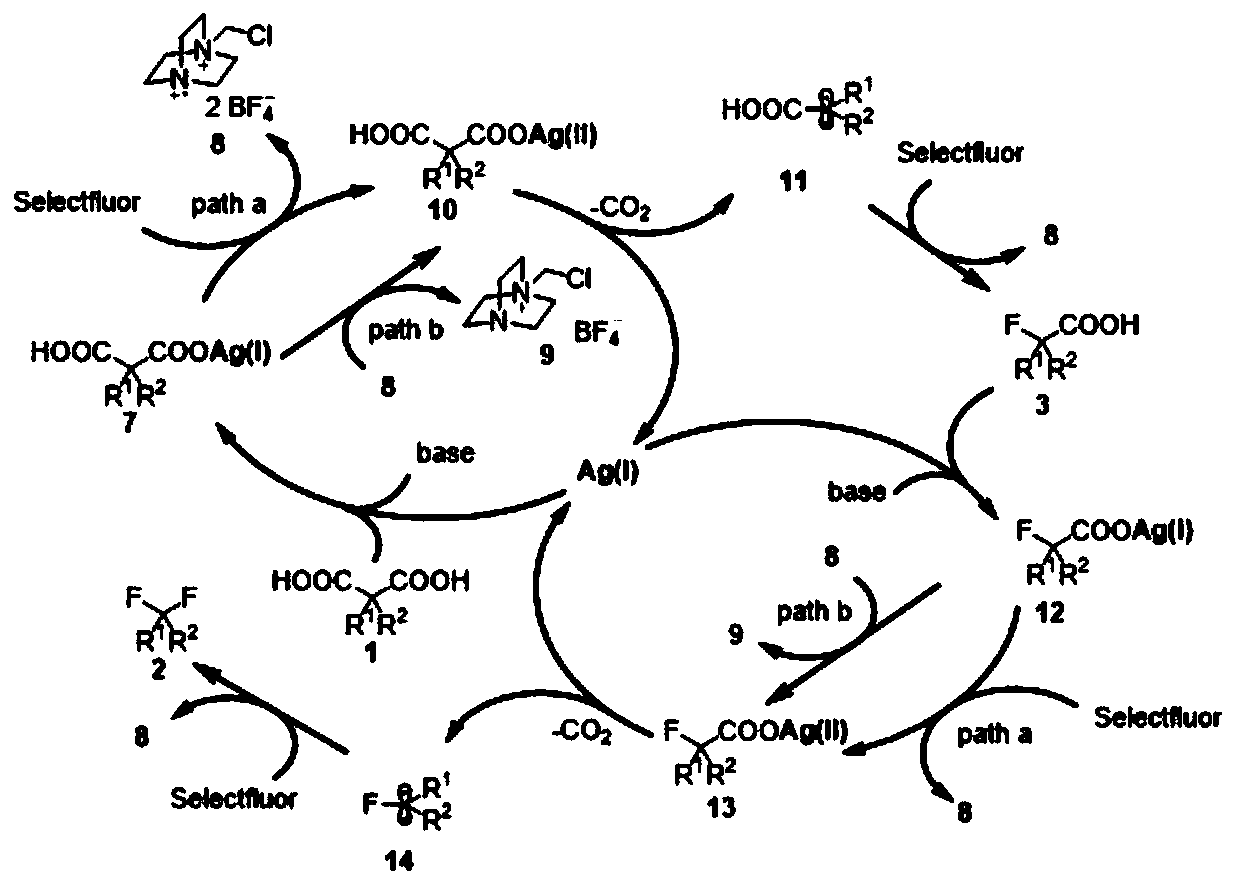
Image
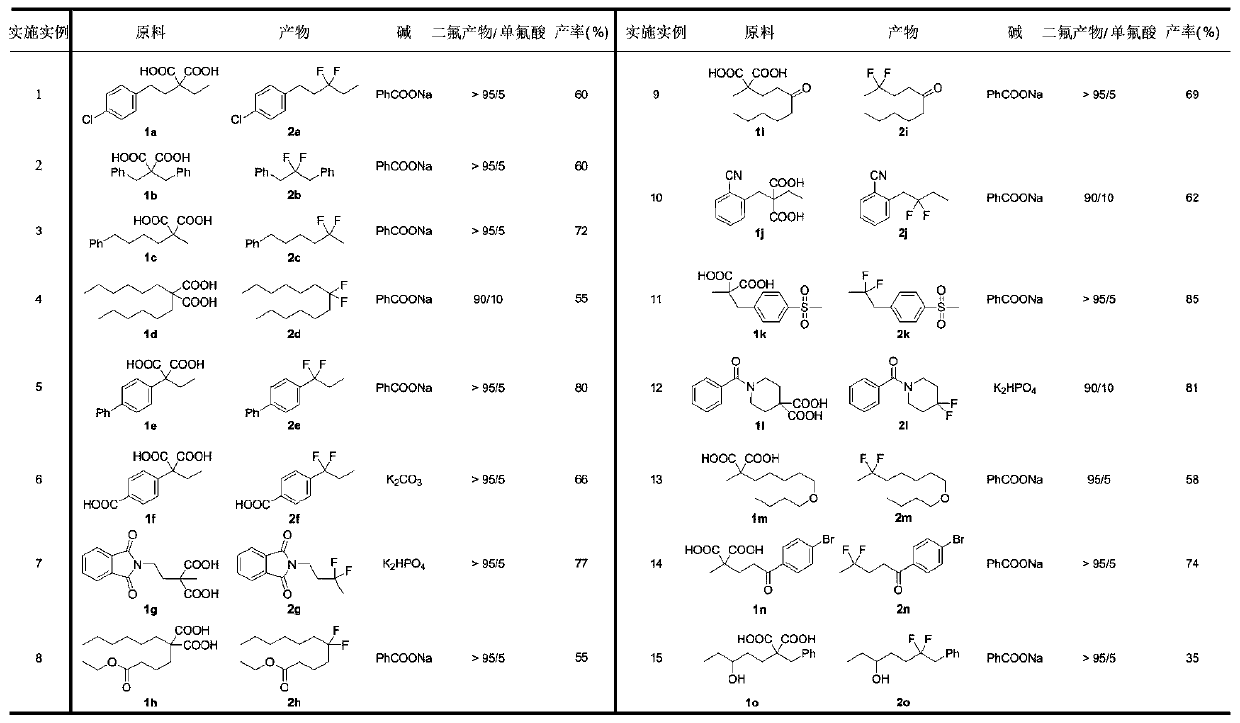
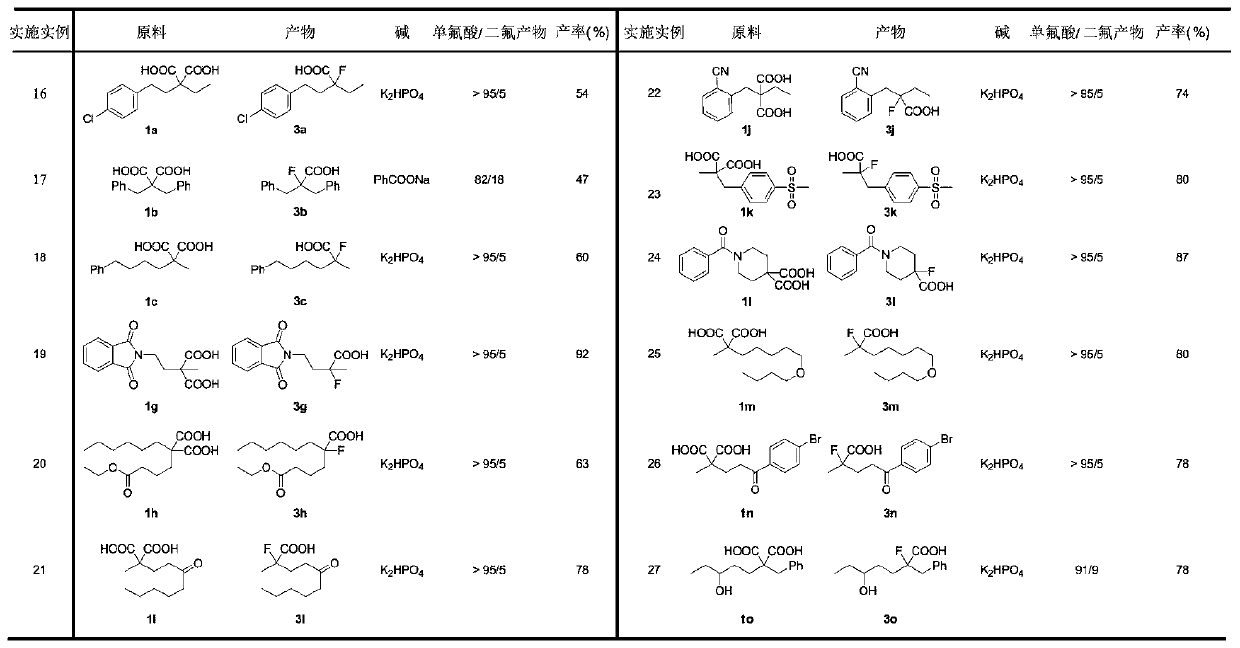
Examples
Embodiment 1
[0036] Synthesis of 1-chloro-4-(3,3-difluoropentyl)benzene (2a)
[0037] 2-(4-Chlorophenyl)-2-ethylmalonic acid (1a, 54.1mg, 0.2mmol), silver nitrate AgNO 3 (10.2mg, 0.06mmol), sodium benzoate PhCOONa (72mg, 0.5mmol), selective fluorine reagent Selectfluor (425.2mg, 1.2mmol), add to the reaction tube, replace N 2 , add acetonitrile / water / n-hexane=1 / 1 / 3 in total 3mL, set the reaction temperature to 55°C, and stir for 12 hours. The reaction was cooled to room temperature, 2 mL of 3M hydrochloric acid solution was added thereto, extracted with ethyl acetate, the organic phases were combined, dried with anhydrous sodium sulfate, filtered, and the organic solvent was spin-dried, and the crude product was purified on a preparative silica gel plate (petroleum ether / acetic acid ethyl ester = 50 / 1). The product 1-chloro-4-(3,3-difluoropentyl)benzene was obtained as a colorless oil, yield: 26.2 mg (60%). 1 H NMR (400MHz, CDCl 3 ):δ7.26(d,J=8.4Hz,2H),7.13(d,J=8.4Hz,2H),2.83–2.72(m,2H...
Embodiment 2
[0039] Synthesis of 2,2-difluoro-1,3-diphenylpropane (2b)
[0040] 2,2-Dibenzylmalonic acid (1b, 56.8mg, 0.2mmol), silver nitrate AgNO 3 (10.2mg, 0.06mmol), sodium benzoate PhCOONa (72mg, 0.5mmol), selective fluorine reagent Selectfluor (425.2mg, 1.2mmol), add to the reaction tube, replace N 2 , add acetonitrile / water / n-hexane=1 / 1 / 3 in total 3mL, set the reaction temperature to 55°C, and stir for 12 hours. The reaction was cooled to room temperature, 2 mL of 3M hydrochloric acid solution was added thereto, extracted with ethyl acetate, the organic phases were combined, dried with anhydrous sodium sulfate, filtered, and the organic solvent was spin-dried, and the crude product was purified on a preparative silica gel plate (petroleum ether / acetic acid ethyl ester = 50 / 1). The product 2,2-difluoro-1,3-diphenylpropane was obtained as a colorless oil, yield: 27.8 mg (60%). 1 H NMR (400MHz, CDCl 3 ):δ7.36–7.16(m,10H),3.09(t,J=16.4Hz,4H). 19 F NMR (376MHz, CDCl 3 ):δ-94.6–-94....
Embodiment 3
[0042] Synthesis of 5,5-Difluorohexylbenzene (2c)
[0043] 2-Methyl-2-(4-phenylbutyl)malonic acid (1c, 50.1mg, 0.2mmol), silver nitrate AgNO 3 (10.2mg, 0.06mmol), sodium benzoate PhCOONa (100.8mg, 0.7mmol), selective fluorine reagent Selectfluor (425.2mg, 1.2mmol), add to the reaction tube, replace N 2 , add acetonitrile / water / n-hexane=1 / 1 / 3 total 5mL, set the reaction temperature to room temperature, and stir for 12 hours. 2 mL of 3M hydrochloric acid solution was added thereto, extracted with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and the organic solvent was spin-dried, and the crude product was purified on a preparative silica gel plate (petroleum ether / ethyl acetate=50 / 1 ). The product 5,5-difluorohexylbenzene was obtained as a colorless oil, yield: 28.5 mg (72%). 1 H NMR (400MHz, CDCl 3 ):δ7.31–7.25(m,2H),7.21–7.15(m,3H),2.63(t,J=7.6Hz,2H),1.93–1.79(m,2H),1.71–1.62(m,2H ),1.62–1.47(m,5H). 19 F NMR (376MHz, CDC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com