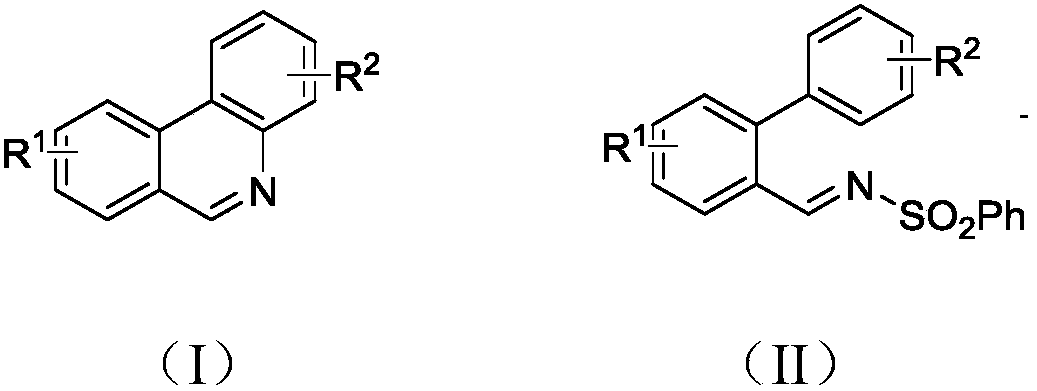
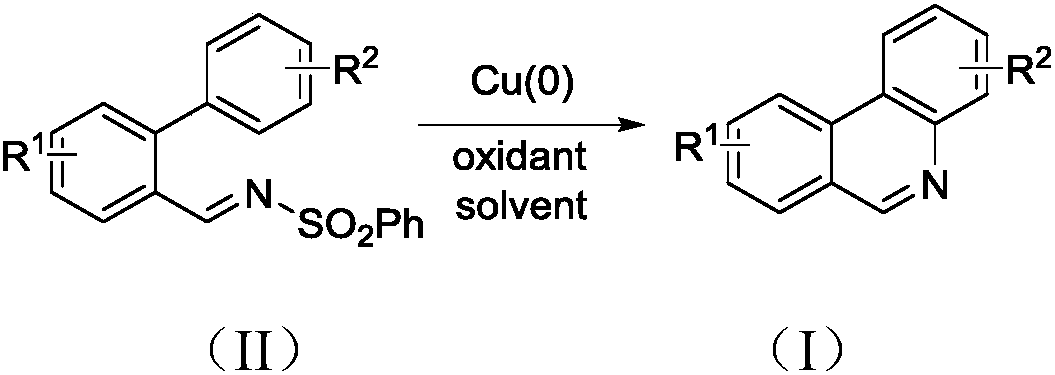
Synthesis method of phenanthridine and derivative of phenanthridine
A synthesis method and technology of derivatives, applied in the field of synthesis of phenanthridine and its derivatives, can solve the problems of not being universally applicable, difficult to obtain reaction raw materials, harsh reaction conditions, etc., and achieve strong substrate universality, economical Effects of energy consumption and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
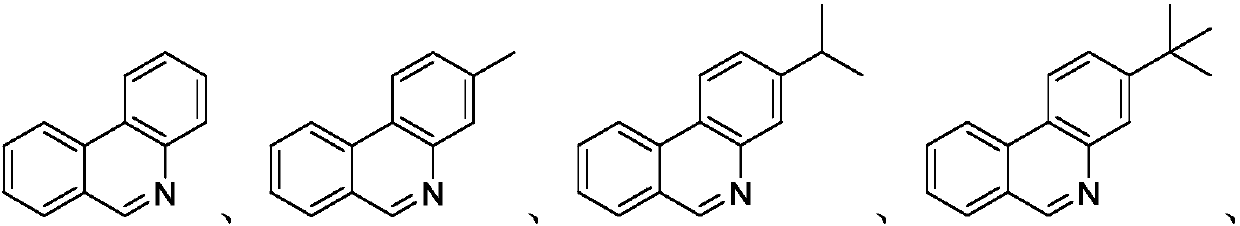
Embodiment 1
[0024]
[0025] Add 96.3mg (0.3mmol) of 2-phenylbenzenesulfonimide, 106.3mg (0.3mmol) of Selectfluor and 1.0mg (0.015mmol) of Cu powder into a 25mL thick-walled pressure-resistant tube, and add 3mL of acetonitrile under nitrogen protection as a solvent. Next, it was magnetically stirred at 50° C. for 12 hours. Then, transfer the reaction solution to a 25mL separatory funnel and add 8mL of dichloromethane and 8mL of water to extract simultaneously, extract 3 times, each time take the organic layer containing dichloromethane and target product, i.e. the extract, and at the end Add 192.6 mg column chromatography silica gel (100-200 mesh) to the extract once, and remove the solvent by distillation under reduced pressure, and separate the residue by column chromatography, and use a mixture of petroleum ether and ethyl acetate with a volume ratio of 10:1 Eluted as an eluent, collected the eluate containing the target product, and distilled off the solvent to obtain a pure produc...
Embodiment 2
[0028]
[0029] Add 100.5mg (0.3mmol) of 2-(4'-methylphenyl)phenylbenzenesulfonimide, 136.2mg (0.6mmol) of DDQ and 1.0mg (0.015mmol) of Cu powder into a 25mL thick-walled pressure-resistant tube 0.9 mL of 1,2-dichloroethane was added as a solvent under the protection of nitrogen. Next, it was magnetically stirred at 50° C. for 12 hours. Then, transfer the reaction solution to a 25mL separatory funnel and add 3mL ethyl acetate and 8mL water to extract simultaneously, extract 3 times, and get the organic layer containing ethyl acetate and the target product every time, i.e. the extract, and at the end Add 502.5 mg column chromatography silica gel (100-200 mesh) to the extract once, and remove the solvent by distillation under reduced pressure, and separate the residue by column chromatography, and use a mixture of petroleum ether and ethyl acetate with a volume ratio of 10:1 Eluted as an eluent, collected the eluate containing the target product, and distilled off the solven...
Embodiment 3
[0032]
[0033]Add 108.9mg (0.3mmol) of 2-(4'-isopropylphenyl)phenylbenzenesulfonimide, 368.9mg (0.6mmol) of Oxone and 1.9mg (0.03mmol) of Cu powder into a 25mL thick-wall pressure-resistant In a separate tube, add 3 mL of 1,4-dioxane as a solvent under nitrogen protection. Next, it was magnetically stirred at 50° C. for 24 hours. Then, transfer the reaction solution to a 25mL separatory funnel and add 12mL of dichloromethane and 8mL of water to extract at the same time, extract 3 times, each time get the organic layer containing dichloromethane and target product, i.e. the extract, and in the last Add 326.7 mg of column chromatography silica gel (100-200 mesh) to the extract, and remove the solvent by distillation under reduced pressure, and separate the residue by column chromatography, using a mixture of petroleum ether and ethyl acetate with a volume ratio of 10:1 as The eluent was eluted, the eluate containing the target product was collected, and the solvent was evap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com