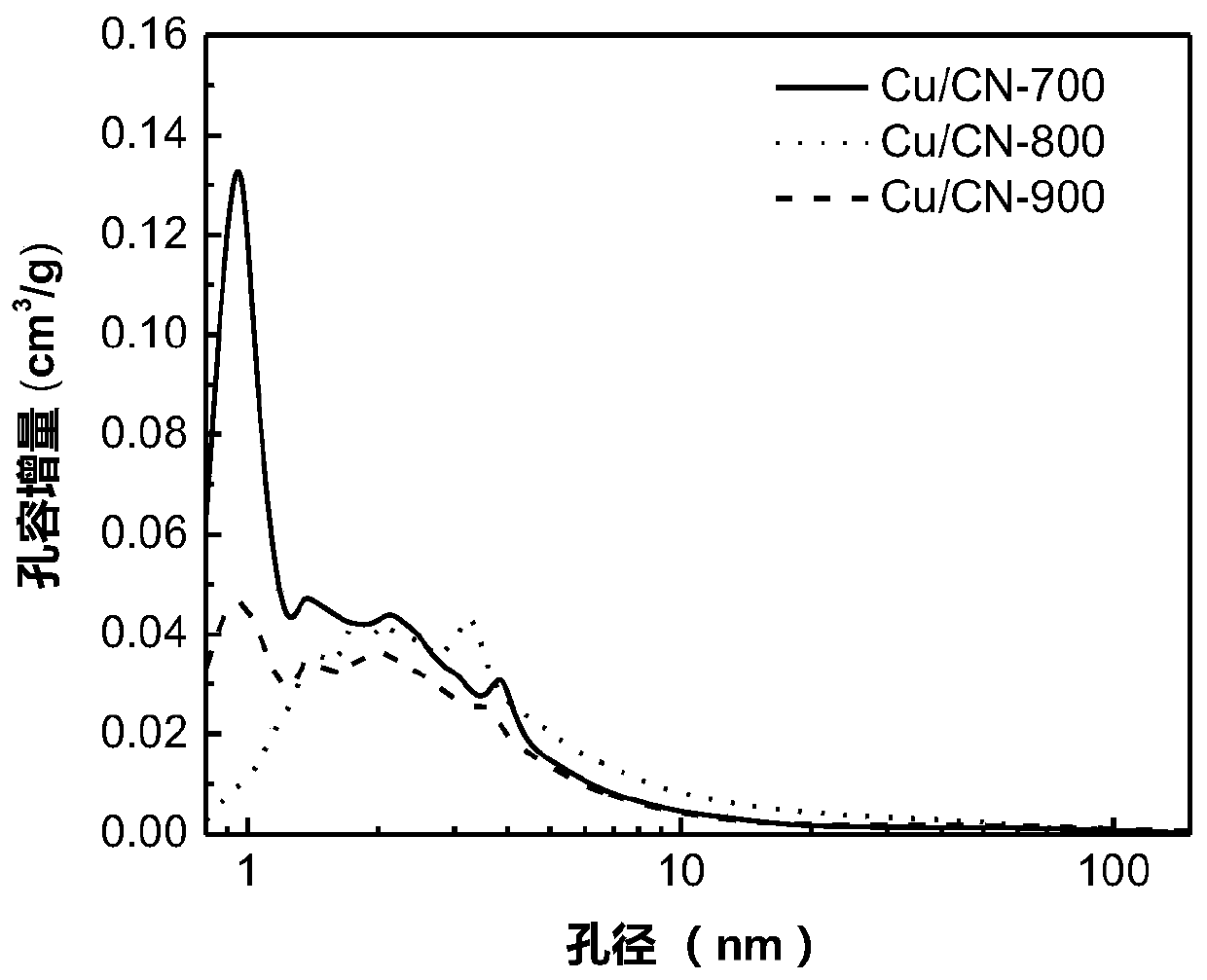
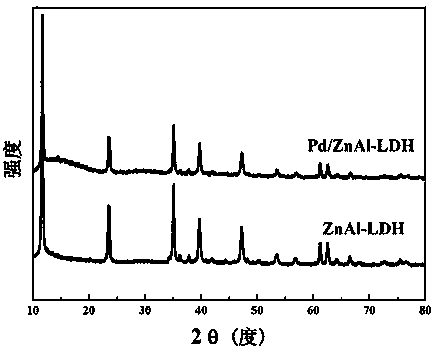
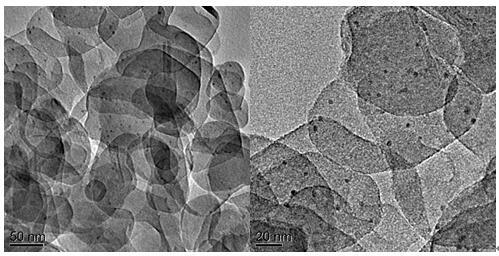
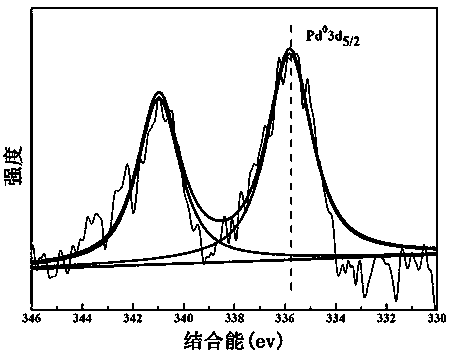
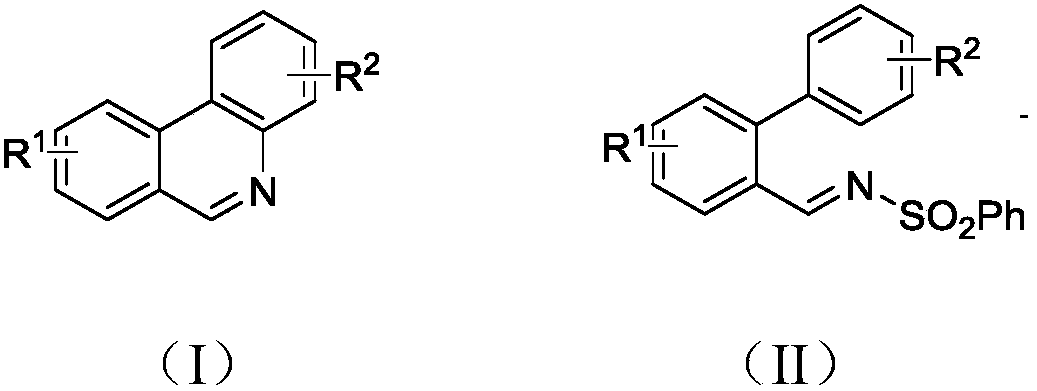
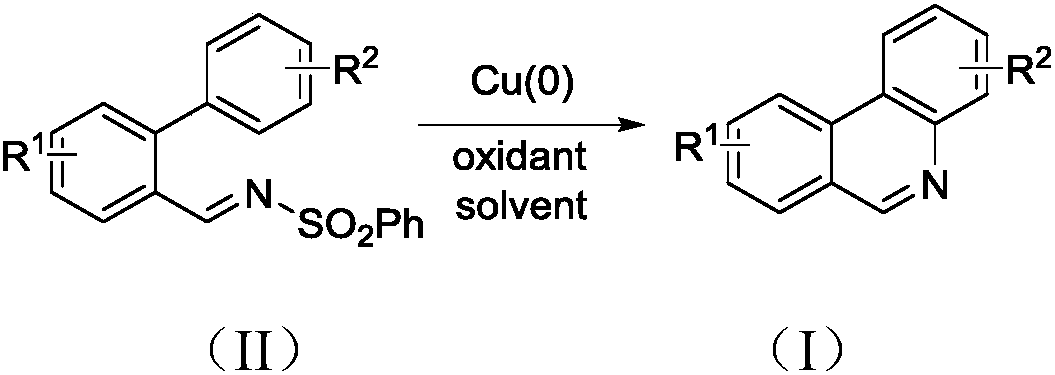
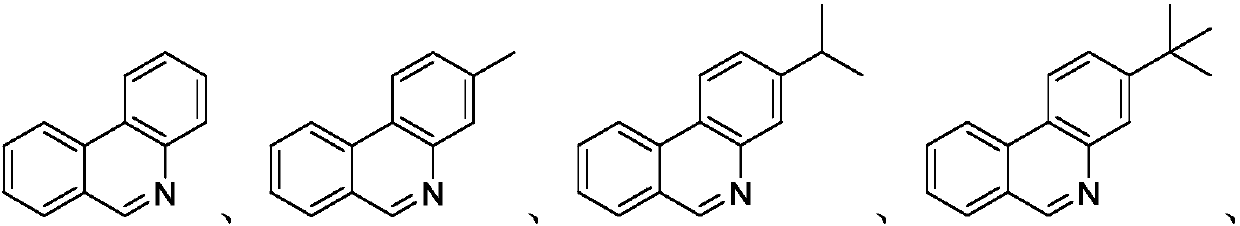
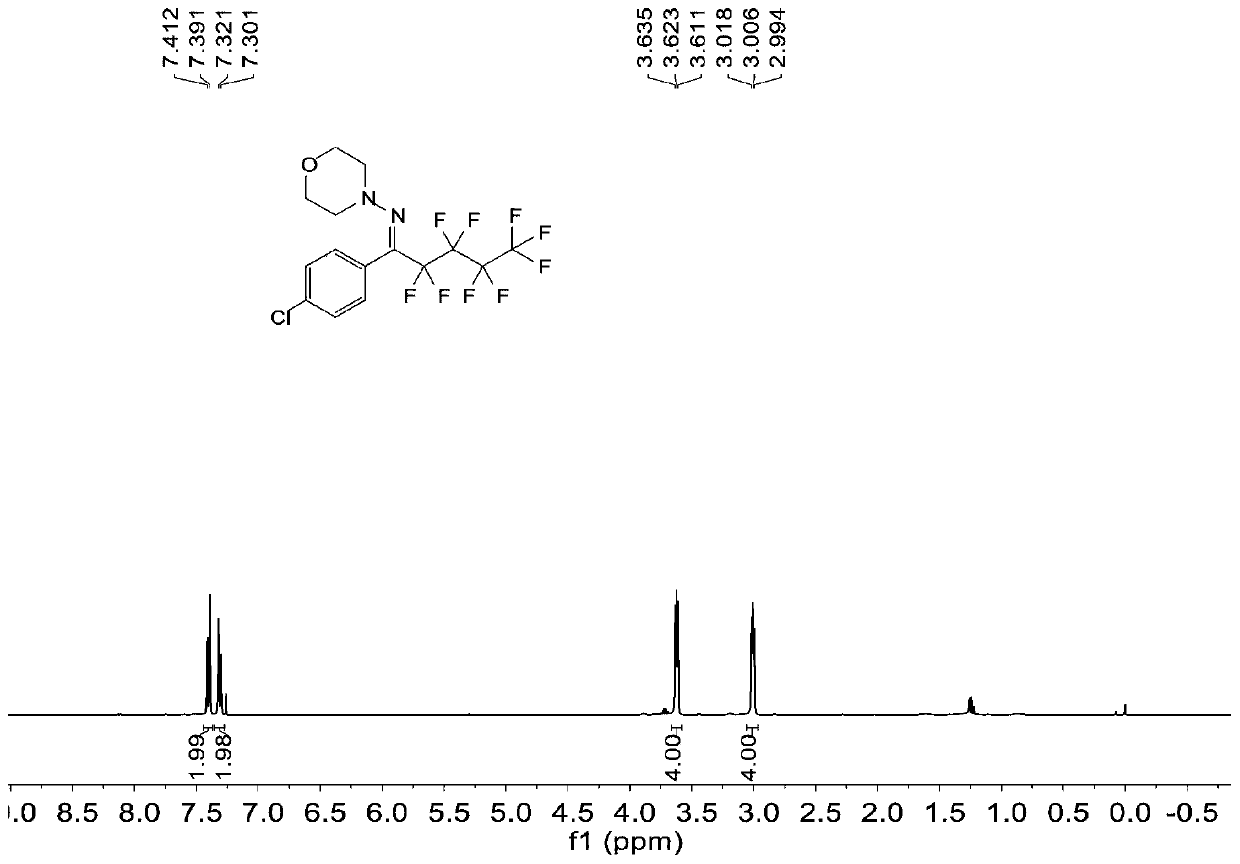
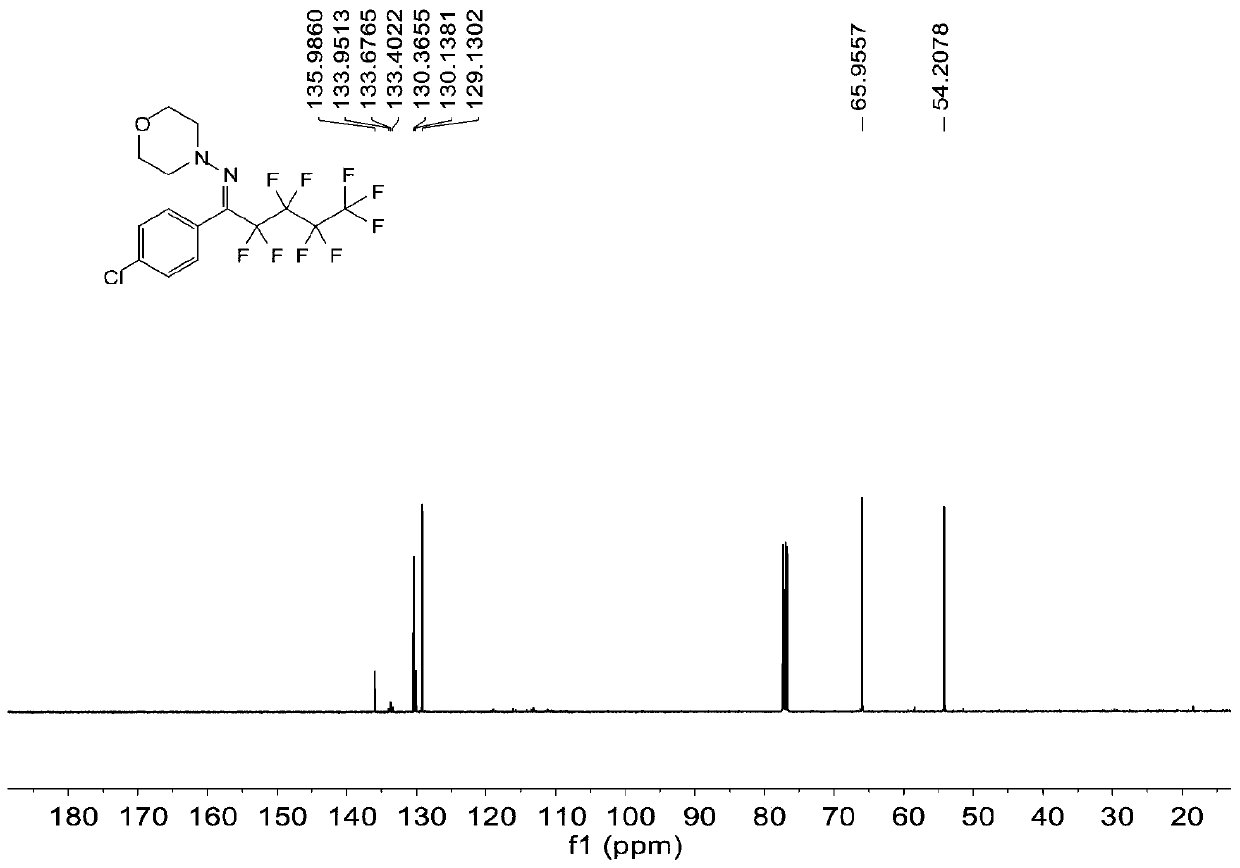
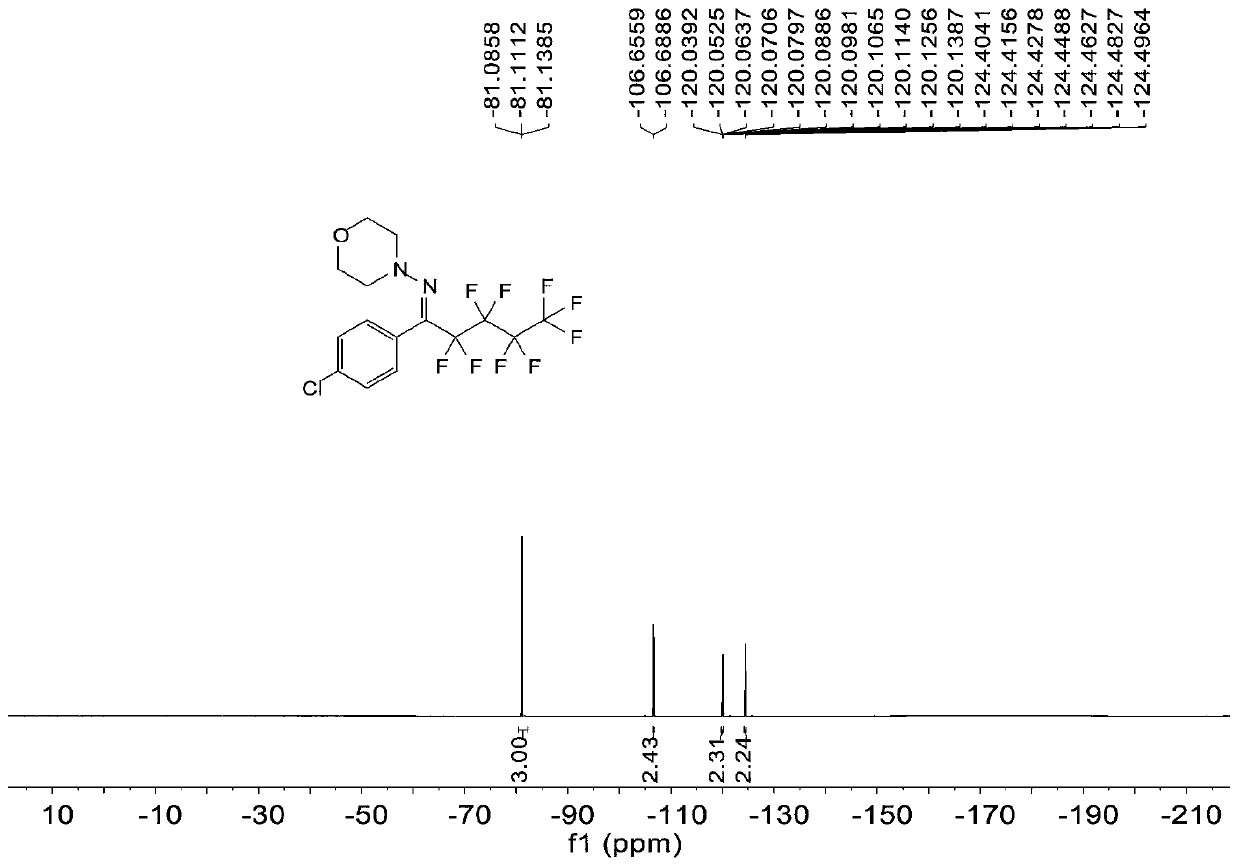
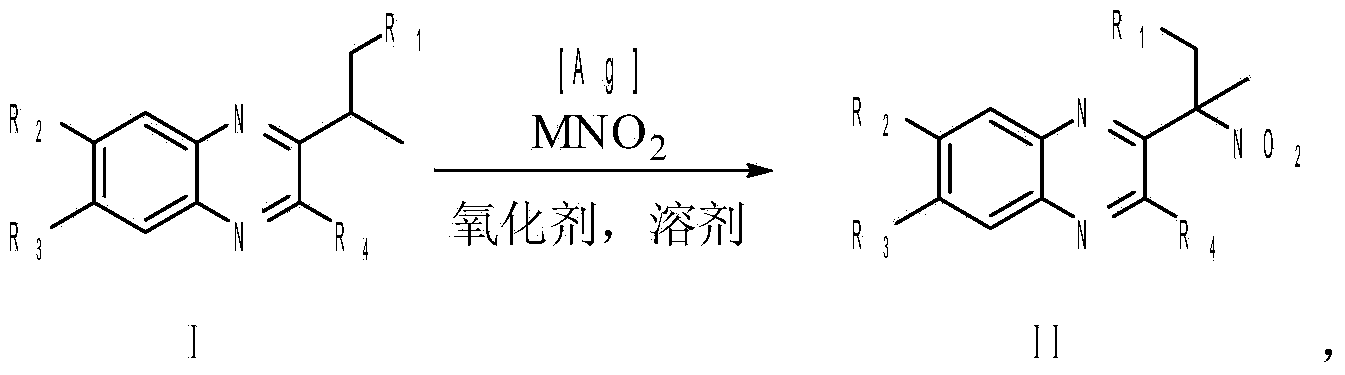
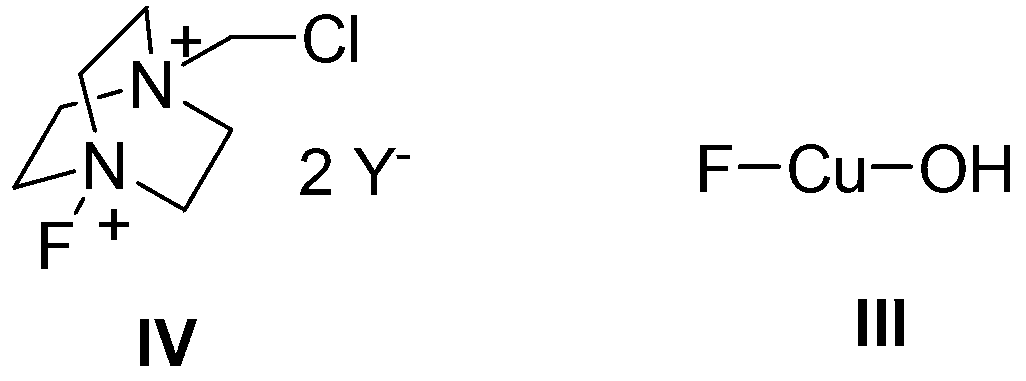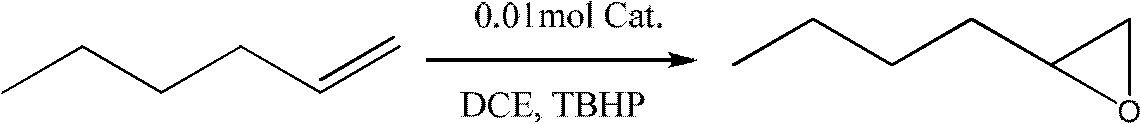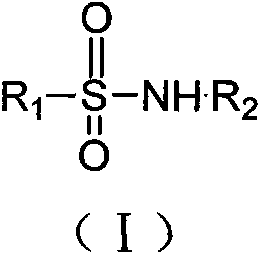Patents
Literature
159results about How to "Good substrate universality" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing ethyl levulinate by catalyzing biomass sugar to be directly alcoholyzed
InactiveCN103724201AHigh yieldUniversalOrganic compound preparationCarboxylic acid esters preparationSugarEthyl levulinate
The invention belongs to the technical field of compound synthesis, and particularly relates to a method for preparing ethyl levulinate by catalyzing biomass sugar to be directly alcoholyzed. According to the method disclosed by the invention, Al3<+> salt is used as a catalyst. The method for preparing the ethyl levulinate by catalyzing biomass sugar to be directly alcoholyzed is not only moderate in reaction condition and strong in substrate universality, but also high in yield of the ethyl levulinate.
Owner:ZHENGZHOU UNIV
Method for synthesizing 2,5-furandimethanol by selective hydrogenation of 5-hydroxymethylfurfural
ActiveCN107442177AHigh acid-base strengthPolyacid-base siteOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsAlcoholHydrogenation reaction
The invention discloses a method for synthesizing 2,5-dihydroxymethylfuran by selective hydrogenation of 5-hydroxymethylfurfural. The method is characterized in that a magnetic metal-organic coordination polymer is used as an acid and base bifunctional catalyst, low-price and easily-obtained low carbon alcohol is used as an in-situ hydrogen donor, and the 5-hydroxymethylfurfural is efficiently transformed into the 2,5-dihydroxymethylfuran by a selective transfer hydrogenation reaction under mild operation conditions and the maximum yield of the 2,5-dihydroxymethylfuran can be up to 98.6 percent. The magnetic metal organic coordination polymer used in the method disclosed by the invention has the advantages of relatively-high acid-base strength, more acid-base sites, relatively-large specific surface area and suitable pore size; in addition, the magnetic metal organic coordination polymer is simple in preparation process, is easy to separate and recover and shows excellent catalytic activity and catalytic stability. Furthermore, the low carbon alcohol is used as the in-situ hydrogen donor in the invention, so that the use of molecular hydrogen is avoided and the safety of the reaction process is improved; besides, the low carbon alcohol can be used as a reaction solvent and the introduction of exogenous substances is reduced, and thereby the production cost can be further reduced.
Owner:HUAIYIN TEACHERS COLLEGE
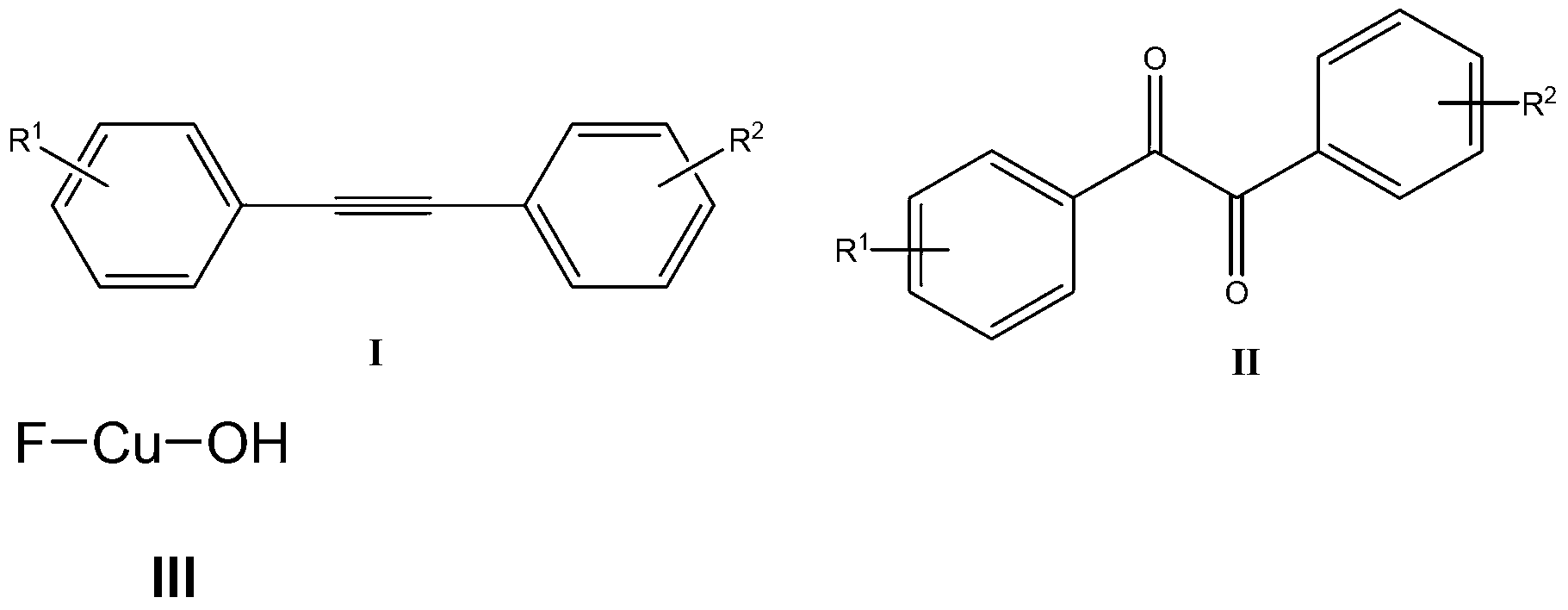
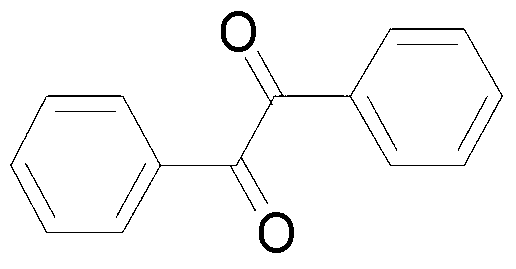
Method for catalyzing and synthesizing benzil derivatives from alkali type copper fluoride
ActiveCN103274917ALow toxicityMild reaction conditionsPhysical/chemical process catalystsCarbonyl compound preparation by oxidationSolventChemistry
The invention provides a method for catalyzing and synthesizing benzil derivatives from alkali type copper fluoride: diphenyl acetylene compounds shown in formula I is the raw material, alkali type copper fluoride is the catalyst, selectflour is the oxidizing agent, mixing acetonitrile and water according to volume ratio of 5-500 to 1 and string the mixture to react at room temperature for 1 to 24 hours, so as to obtain the benzil derivatives shown in formula II by post processing and preparing of the reaction liquid after reaction; and alkali type copper fluoride is prepared by mixing copper powder, 1-Chloromethyl-4-fluoro-1 and 4 - dinitrogen mixed double loop (2.2.2) octane salt and reacting under the action of the mixed solution of organic solvent and water. The method provided by the invention has the advantages that the catalyst is low in cost and toxicity, the oxygen source is easy to get and environmental friendly, the reaction condition is moderate, the functional group universality is good, and the operation is simple and convenient.
Owner:YANGZHOU JUNRUI CHUANGZHI IND DESIGN
Biomass-based carbon material loaded monatomic copper catalyst as well as preparation method and application thereof
ActiveCN111068682AEasy to prepareAvoid gatheringCarboxylic acid nitrile preparationOrganic compound preparationArylPtru catalyst
The invention discloses a biomass-based carbon material loaded monatomic copper catalyst and a preparation method thereof, and a method for synthesizing a 1, 3-diyne compound by oxidative coupling ofthe catalyst. The catalyst is composed of 0.01 wt%-3 wt% of metal monatomic copper, 70 wt%-90 wt% of a carbon-based carrier and 5 wt%-20 wt% of heteroatoms. The preparation method of the biomass-basedcarrier-loaded monatomic copper catalyst is simple, mild in condition and low in cost, and does not need a common subsequent pickling process. The cheap metal salt and self-doped heteroatom N are used for synergistic coordination, aggregation of metal in the high-temperature pyrolysis process is avoided, and monodispersion of metal atoms is achieved. The metal monatomic content is high, the dispersion is uniform, and the physicochemical structure is stable. The catalyst can take air as an oxidizing agent in oxidative coupling reaction, achieves high conversion rate and excellent substrate universality under alkali-free and ligand-free conditions, and realizes an oxidative cross-coupling reaction among aryl-aryl, aryl-alkyl and alkyl-alkyl differently substituted substrates.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Hydrotalcite-loaded palladium catalyst for preparing styrene through selective hydrogenation of phenylacetylene and preparation method thereof and application
ActiveCN111054333AEasy to manufactureSuitable for mass productionOrganic reductionOrganic compound preparationPolymer sciencePtru catalyst
The invention discloses a hydrotalcite-loaded palladium catalyst for preparing styrene through selective hydrogenation of phenylacetylene and a preparation method thereof and application. According tothe catalyst, hydrotalcite is used as a carrier, and palladium is dispersed on the surface of the carrier in a nanoparticle form. The carrier is any one of ZnAl, NiAl, CoAl and NiFe hydrotalcite. Theactive component is palladium, and the mass fraction of palladium is 0.5-1.5%. The preparation method comprises the following steps of: dispersing hydrotalcite in mixed alcohol, adding a noble metalsalt solution, stirring for several hours, centrifuging and drying to obtain the hydrotalcite-loaded palladium nano-catalyst. The preparation method of the catalyst is simple and suitable for large-scale production. The obtained catalyst has extremely high activity and styrene selectivity in a selective hydrogenation reaction of phenylacetylene, and also has good stability and substrate universality.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Solid acid catalyst and application thereof to synthesis of reproducible diesel oil or aviation kerosene
ActiveCN104971775ARaw materials are cheap and easy to getThe synthesis method is simpleOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsAlkaneCross-link
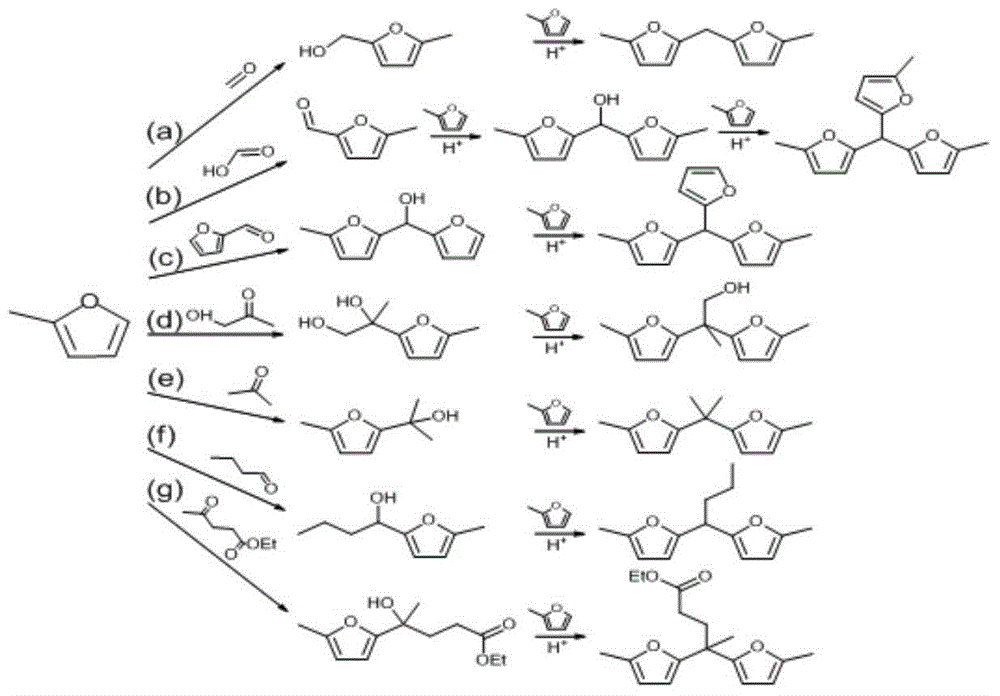
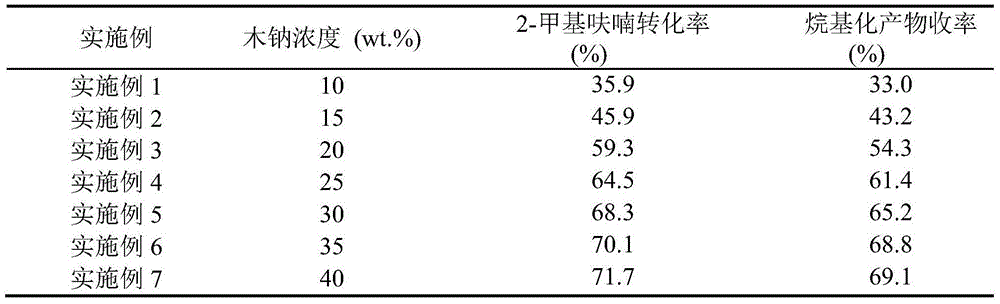
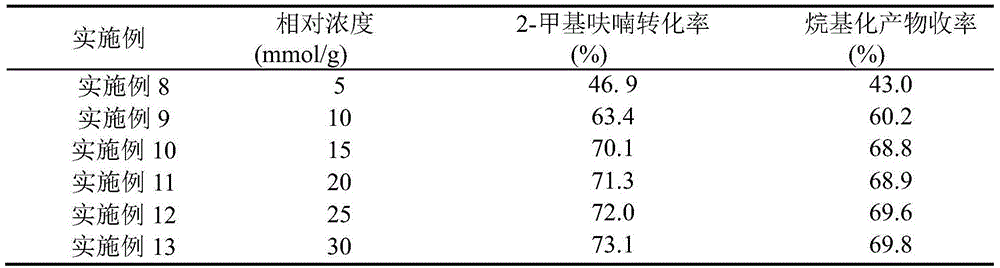
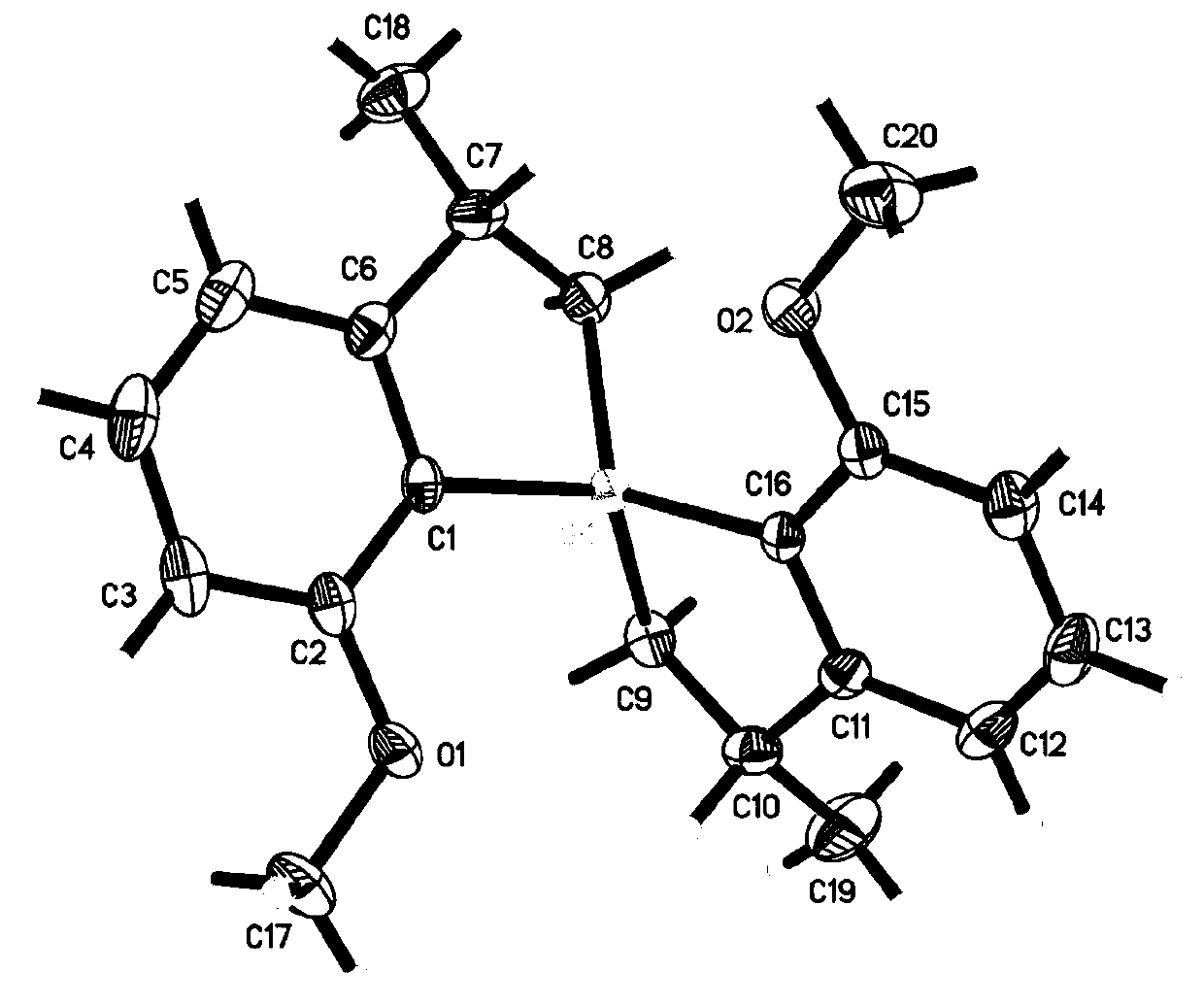
The invention relates to preparation of a solid acid catalyst and application of the solid acid catalyst to an alkylation reaction between lignocellulose-based platform compounds. The preparation method of a sodium lignin sulfonate derived phenolic aldehyde resin solid acid catalyst, provided by the invention is commonly divided into two steps: (1) adding a carbonyl compound into a sodium lignin sulfonate water solution to be used as a cross-linking agent; adding a phenol functional group in acid catalyzed sodium lignin sulfonate and carbonyl compound into the mixture to be subjected to a phenolic aldehyde condensation reaction to obtain a high-molecular polymer difficultly dissolved in water; and (2) carrying out ion exchange on a condensation product generated by the step 1 to obtain an acidic resin material containing sulfonate groups. According to the catalyst, the raw materials are cheap and easily obtained and the preparation process is simple; and the catalyst has very high catalytic activity and selectivity on the alkylation reaction between a lignocellulose-based furan compound and the carbonyl compound without solvents. An alkylated product obtained by the reaction is hydrogenated and deoxygenized to obtain the diesel oil or the aviation kerosene. The cheap and efficient solid acid catalyst, which is used for synthesizing a diesel oil or aviation kerosene precursor, by the lignocellulose-based platform compounds is provided by the invention.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
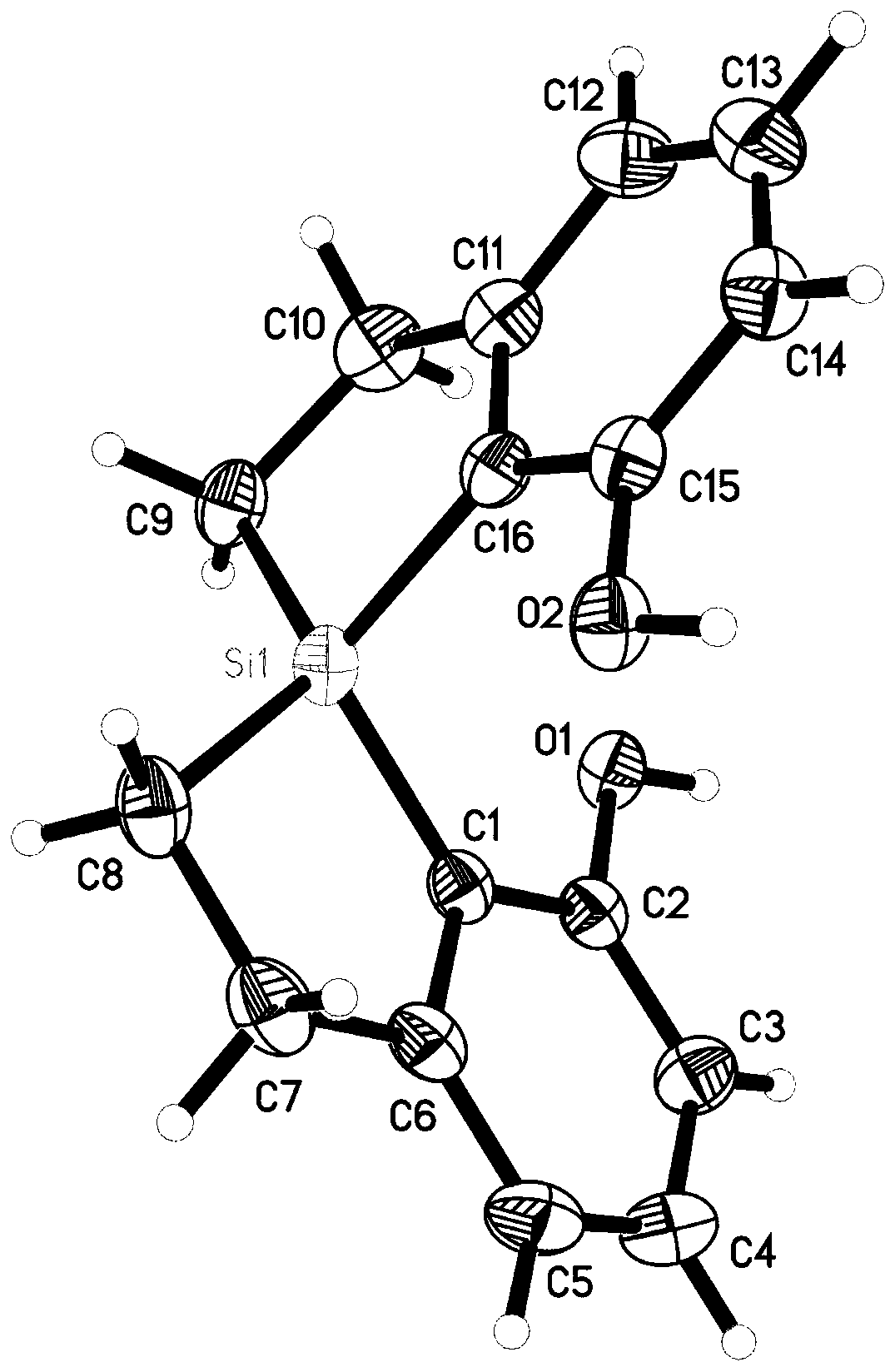
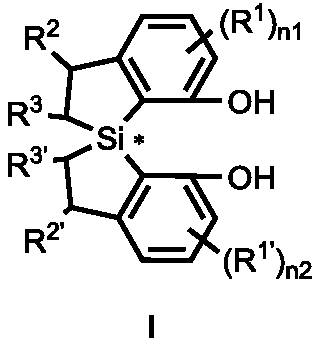
Spirobi(dihydrobenzosilole) diphenol compound, and synthesis method and application thereof
ActiveCN111217848AExtend the research processSimple and fast operationSilicon organic compoundsOrganic compound preparationOrganic synthesisPhenol Compound
The invention discloses a spirobi(dihydrobenzosilole) diphenol compound represented by formula I shown in the specification, and a synthesis method and an application thereof, and concretely providesa method for efficiently constructing the spirobi(dihydrobenzosilole) diphenol compound represented by formula I by using cheap and easily available initial raw materials. The method has the characteristics of simplicity and convenience in operation, easily available raw materials, mild reaction conditions, high yield, convenience in purification and the like. The spirobi(dihydrobenzosilole) diphenol compound of the formula I can be directly used as an organic catalyst, or can be used together with group III to group XIII metal salts to generate a metal complex or be used in an in-situ mixed manner to catalyze an asymmetric organic synthesis reaction; and the compound has the advantages of realization of excellent yield and stereospecific conversion under mild conditions, good substrate universality, and great expansion of the research process of asymmetric catalysis.
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
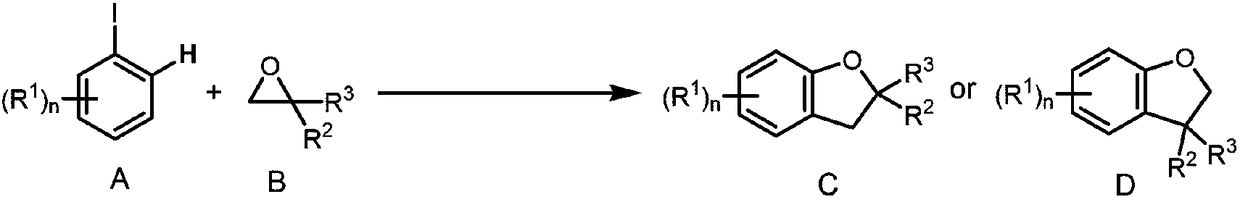
Method for synthesizing 2,3-dihydrobenzofurans compound
InactiveCN108329285ANo special handling requiredLow priceGroup 4/14 element organic compoundsSteroidsOrganic solventIodide
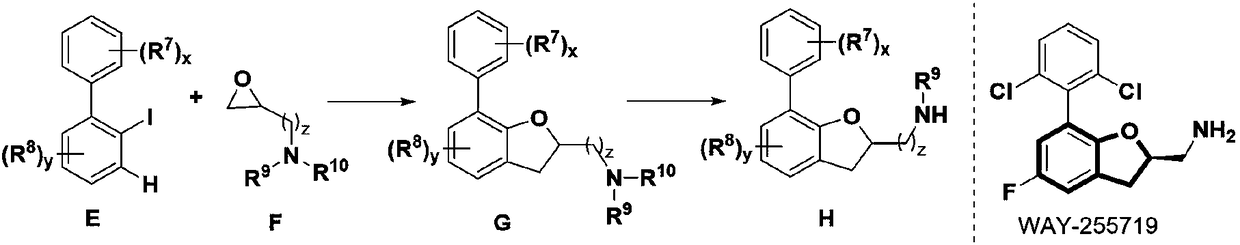
The invention provides a method for synthesizing a 2,3-dihydrobenzofurans compound. The method comprises the following steps: dissolving aromatic iodide, an epoxy compound, a palladium catalyst, a phosphine ligand and a norborene derivative in an organic solvent together; then carrying out stirring reaction at the temperature of 30-120 DEG C; and carrying out separation and purification after reaction to obtain the 2,3-dihydrobenzofurans compound. By the method, the 2,3-dihydrobenzofurans compound can be synthesized efficiently, economically and environmentally friendly. The method is gentle in condition, good in substrate universality and high in yield, and the prepared 2,3-dihydrobenzofurans compound is widely applied to the fields of medicinal chemistry and organic chemistry.
Owner:WUHAN UNIV
Organic reaction catalyst based on shrimp and crab scraps and preparation method and applications thereof
ActiveCN108940373AExtensive sources of raw materialsLow costCarboxylic acid nitrile preparationOrganic compound preparationOrganic reactionOrganocatalysis
The invention discloses an organic reaction catalyst based on shrimp and crab scraps and a preparation method and applications thereof. The catalyst is prepared from shells of shrimps and crabs. The preparation method comprises following steps: (1) drying shrimp and crab scraps, and then grinding shrimp and crab scraps to obtain shrimp and crab powder; (2) adding the shrimp and crab powder into astrong alkali solution, and heating the strong alkali solution to remove proteins and acetyl groups; and (3) after the strong alkali treatment, filtering the shrimp and crab powder, washing, and drying to obtain a chitosan based organic catalyst containing a calcium carbonate carrier. The provided organic catalyst has following advantages: (1) the raw material sources are wide, the cost is low, and the raw materials are easily available; (2) the prepared catalyst has the advantages of high catalytic activity, large specific surface area, easy recovery, and good substrate universality; and (3)the preparation method has the advantages of simple operation and easy industrialization.
Owner:广东道科特生物科技有限公司
Method for synthesizing 3-aryl thiopropionamide derivative
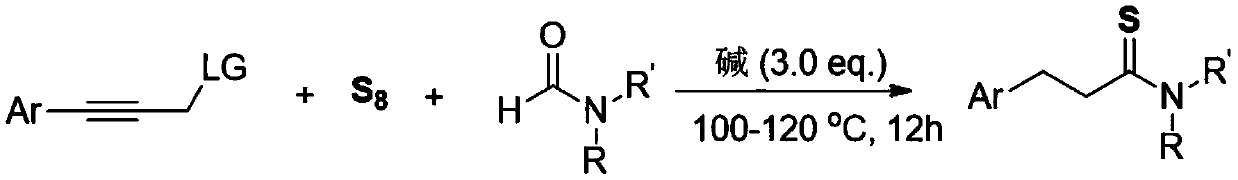
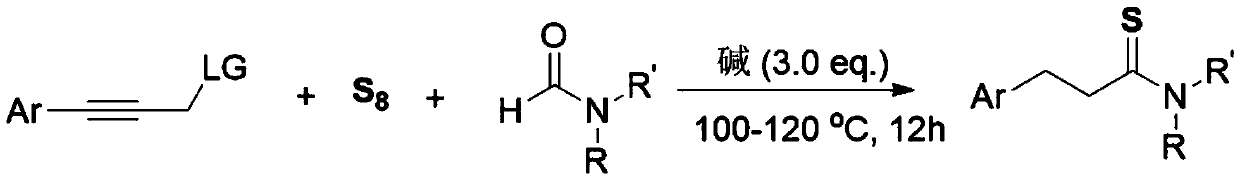
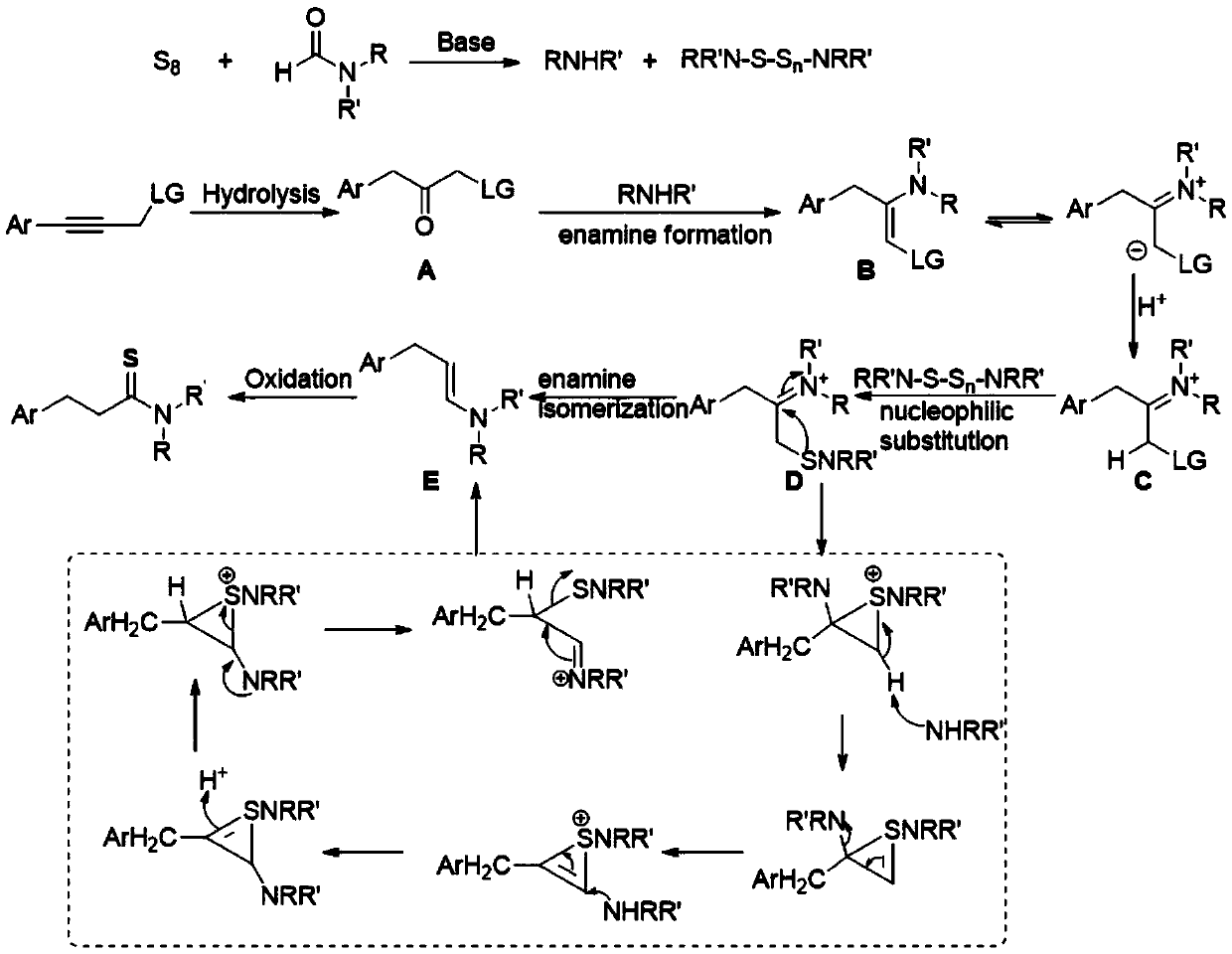
The invention relates to a method for synthesizing a 3-aryl thiopropionamide derivative. The method comprises the following step: by taking an aryl allylene derivative as a substrate, an alkali as a promoter and elemental sulfur as a sulfur source, performing a stirring reaction for 12 hours in a formamide solvent at 100-120 DEG C. The method adopts the elemental sulfur which is odorless, easy toobtain and low in price as the sulfur source, and has the advantages of being simple and easy in raw material obtaining, simple in reaction operation, mild in condition, wide in substrate universality, high in yield and good in functional group compatibility.
Owner:WENZHOU UNIVERSITY
Synthesis method of phenanthridine and derivative of phenanthridine
InactiveCN107778239AReduce consumptionRaw materials are easy to obtainOrganic chemistryOrganic solventSynthesis methods
The invention discloses a synthesis method of phenanthridine as shown in a formula (I) and a derivative of the phenanthridine. In the synthesis method, o-arylphenylsulfimide shown as a formula (II) isused as a raw material and reacts in an organic solvent under the action of a [Cu] / Selectfluor catalyst to obtain a corresponding target product (I). The synthesis method disclosed by the invention has the advantages of cheap and easily available and low-toxicity catalyst, environmental friendliness, mild reaction conditions, high universality of functional group and easiness and convenience in operation. The formulas (I) and (II) are shown in the description.
Owner:ZHEJIANG UNIV OF TECH
Glyceryl solid acid catalyst and application thereof
ActiveCN104941680ARaw materials are cheap and easy to getThe synthesis method is simpleOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsFuranAlkane
The invention relates to an application of a solid acid catalyst taking glycerol as a raw material in synthesis of reproducible diesel or aviation kerosene. A preparation method of the glyceryl solid acid catalyst, disclosed by the invention, comprises the following steps: uniformly mixing glycerol and concentrated sulfuric acid in a certain proportion, enabling a mixture to react for a certain period of time at a certain temperature, and filtering and washing a solid product obtained by reaction to obtain the catalyst. In a glycerol and sulfuric acid reaction process, a silicon oxide hard template can be added and removed by using HF after reaction, so that the performance of the catalyst can be remarkably improved. According to the invention, material of the catalyst is cheap and easily-available, the preparation process is simple, and relatively high activity and selectivity can be realized on alkylation reaction between lignocellulose base furan compounds and a carbonyl compound. Diesel or aviation kerosene alkane can be obtained after an alkylate product obtained by reaction is subjected to hydrodeoxygenation. The invention provides a cheap and efficient catalyst for synthesizing a diesel or aviation kerosene precursor by virtue of a lignocellulose platform chemical compound.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing acetyl propionate from biomass sugar
InactiveCN104693023AOvercoming productivity issuesHigh yieldOrganic compound preparationCarboxylic acid esters preparationPropionateAlcohol
The invention discloses a method for preparing acetyl propionate from biomass sugar. The method comprises the following steps: reacting for 3-6 hours in an alcohol solution of 190-210 DEG C by taking the biomass sugar and magnetic zirconium phosphate solid acid as a catalyst to obtain acetyl propionate, wherein a molar ratio of P to Zr in the magnetic zirconium phosphate solid acid is 1-3, the alcohol is methanol, alcohol, propyl alcohol or butyl alcohol; a mass ratio of the magnetic zirconium phosphate solid acid to the biomass sugar is (0.4-2) to 1. According to the method disclosed by the invention, raw materials are renewable resources, and the acetyl propionate is directly prepared by adopting the magnetic solid acid which is easy to prepare, easy to separate and capable of recycled through catalysis one-step process. The method is simple in process, safe to operate, relatively low in equipment requirement and low in production cost, and is an environment-friendly production process.
Owner:XIANGTAN UNIV
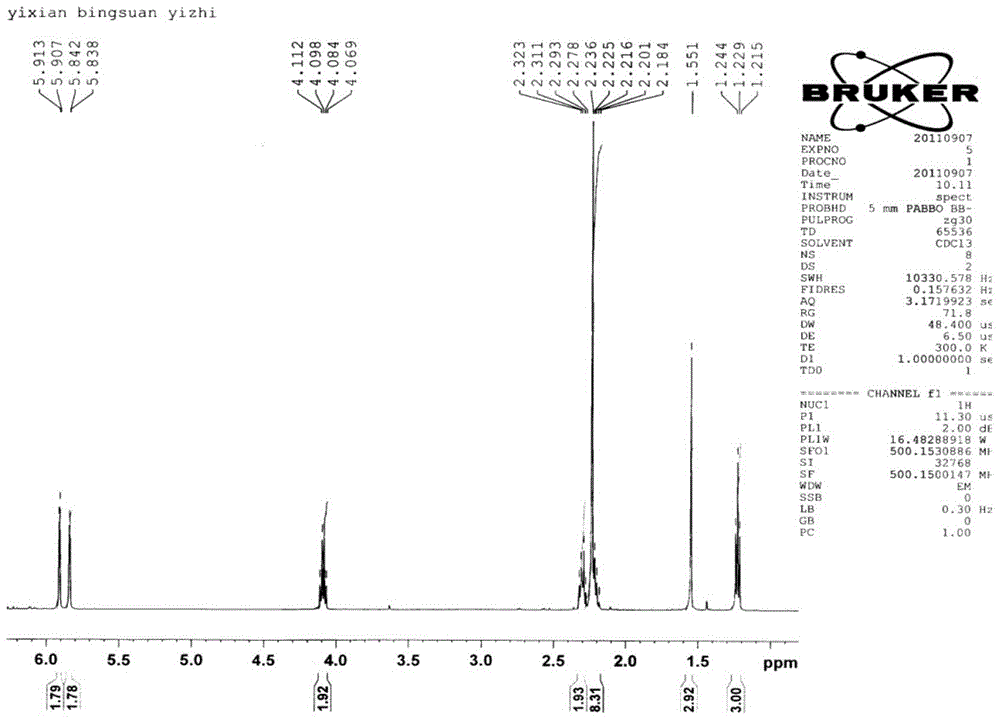
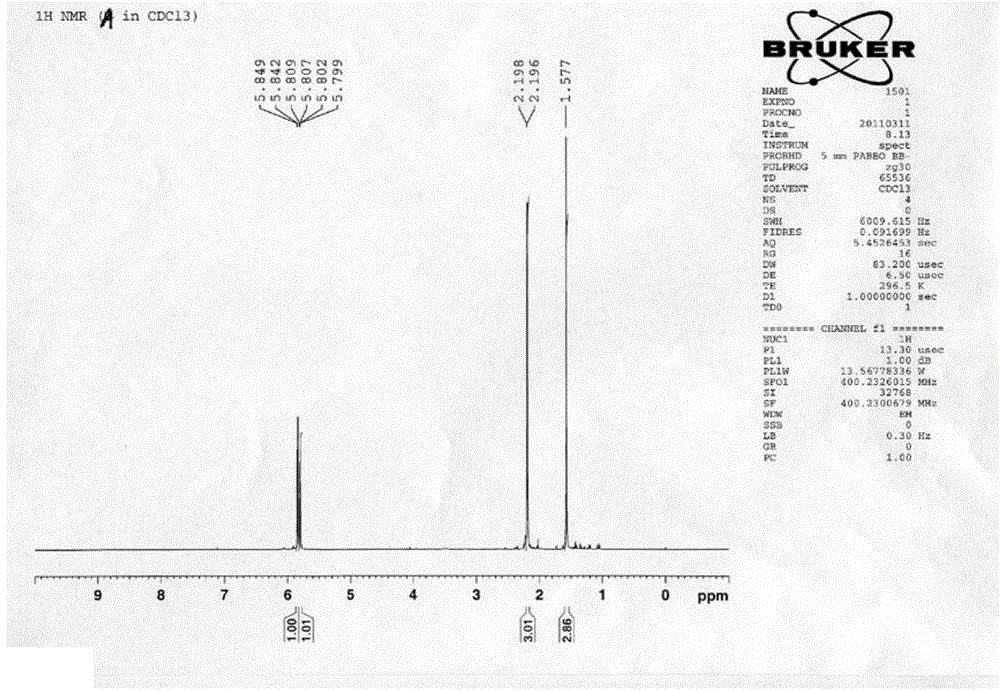
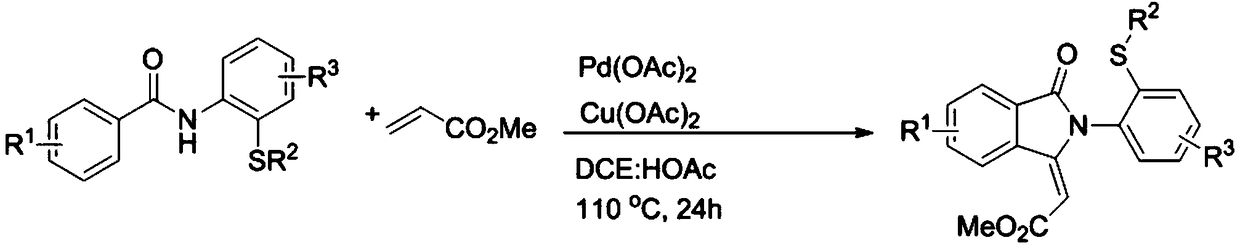
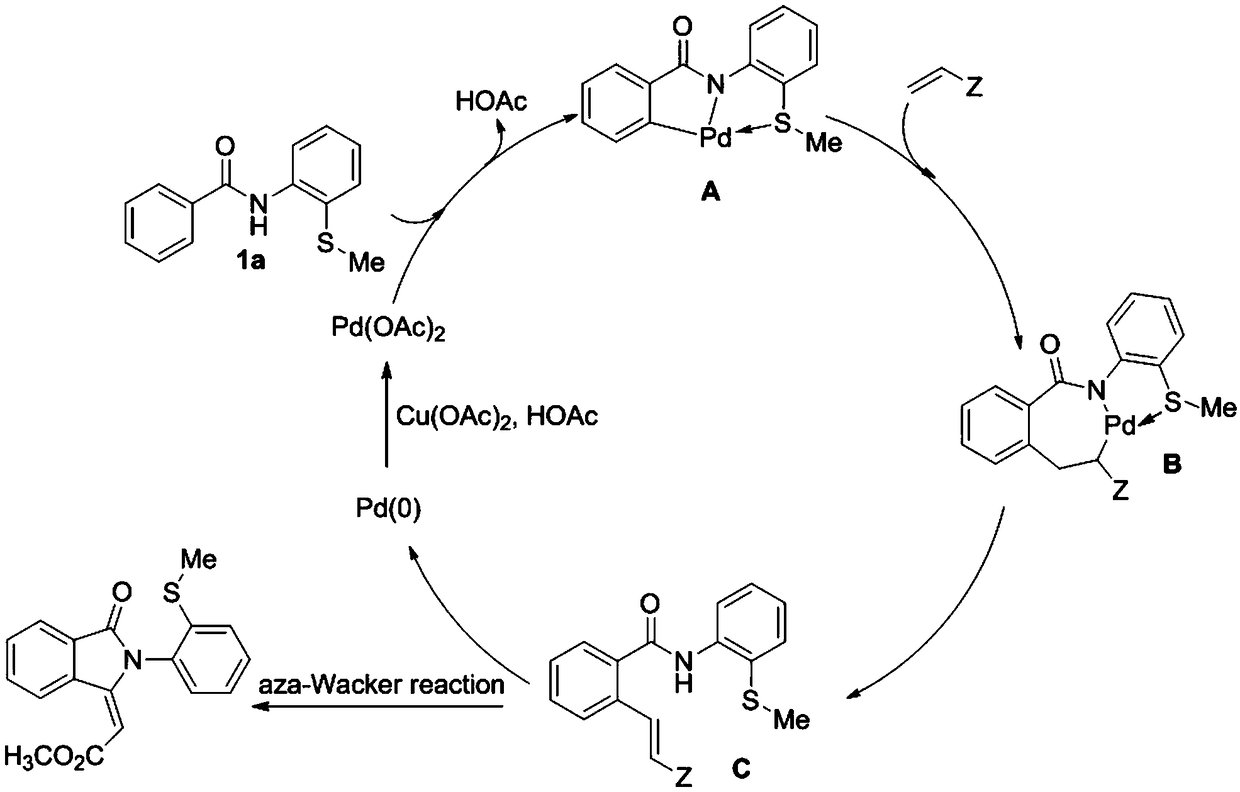
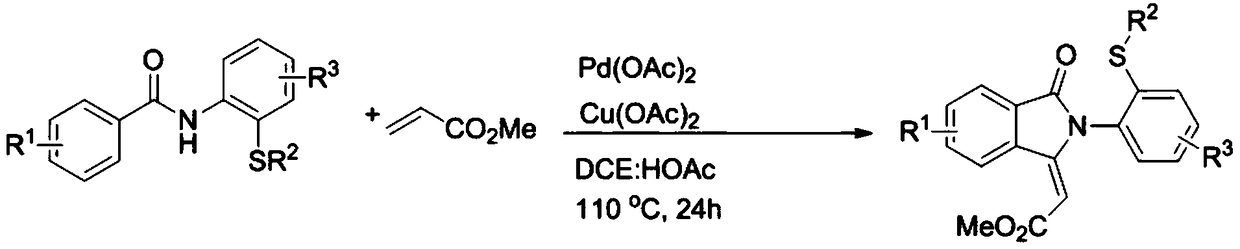
Preparation method for sulfur-containing 3-methylene isoindoline-1-one derivative
ActiveCN109369504ASimple processSimple and fast operationOrganic chemistrySodium bicarbonateOrganic layer
The invention relates to a preparation method for sulfur-containing 3-methylene isoindoline-1-one. The preparation method comprises the steps that N-(2-(methylthio)phenyl) benzamide and methyl acrylate serve as substrates, Pd(OAc)2 serves as a catalyst, anhydrous copper acetate serves as an oxidizing agent, a mixture is put into a mixed solution of 1,2-dichloroethane and glacial acetic acid in anoil bath at 110 DEG C for reaction for 24 hours, after the reaction is ended, a solvent of the glacial acetic acid of the mixture is neutralized by using a sodium bicarbonate saturated solution, thena water layer is extracted by using ethyl acetate, a combined organic layer is dried by using anhydrous magnesium sulfate, the solvent is removed under reduced pressure, and residues are purified through a rapid column chromatography method so as to obtain a required product. The preparation method has the advantages that the raw materials are simple and easy to obtain, a preparation process is novel and simple, the pollution is less, the energy consumption is low, and the yield is high.
Owner:WENZHOU UNIVERSITY
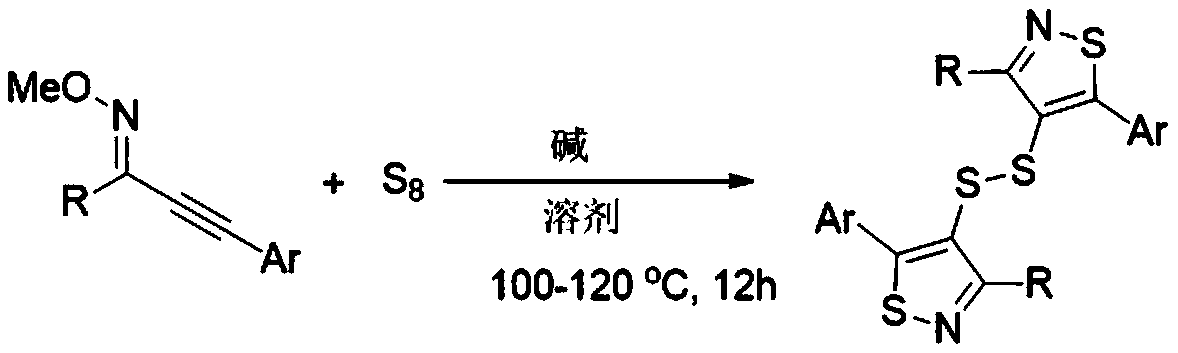
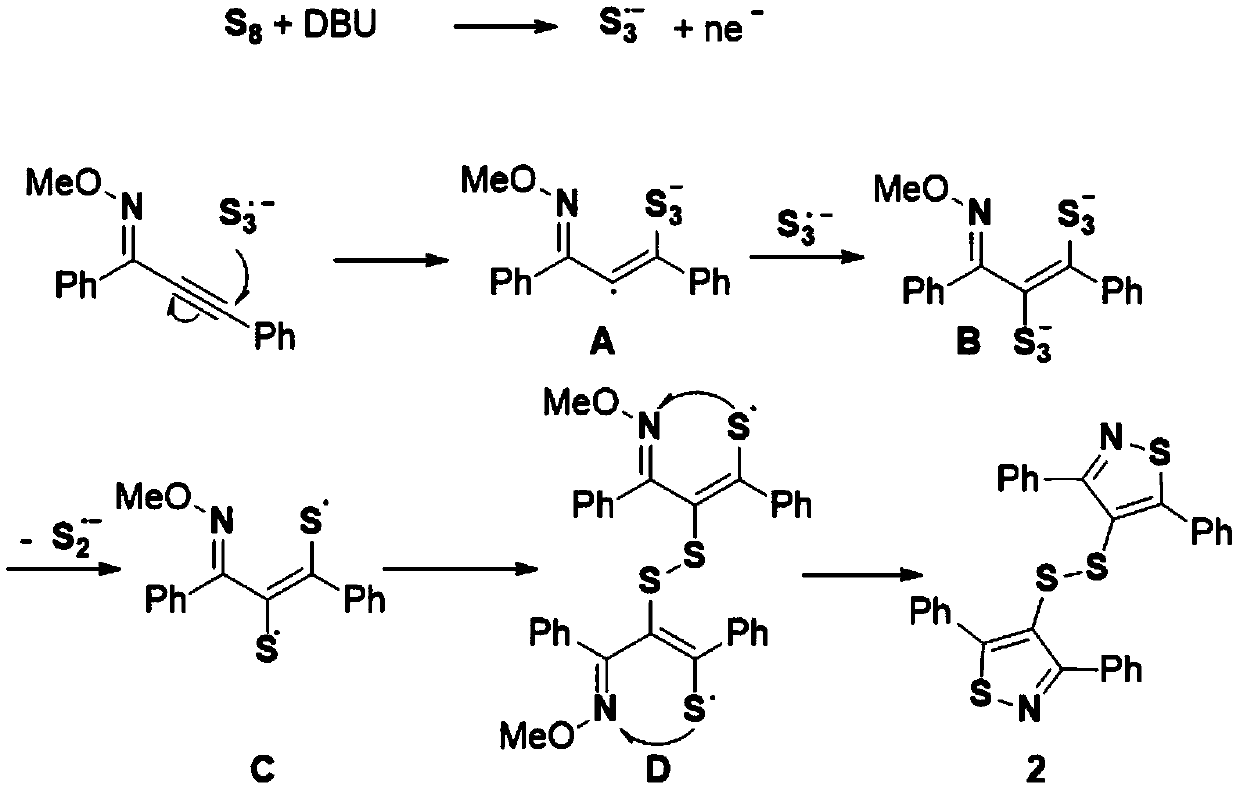
Synthesis method of bithiazole-4-yl disulfide derivative
ActiveCN111233783ASimple processSimple and fast operationOrganic chemistryThiazoleCombinatorial chemistry
The invention relates to a synthetic method of a bithiazole-4-yl disulfide derivative. The synthesis method comprises the following steps: taking alkynyl oxime ether as a substrate, taking elemental sulfur as a sulfur source, taking 1,8-diazabicycloundec-7-ene as alkali and NMP-H2O (5:1, V:V) as a solvent, and carrying out a reaction at 100-120 DEG C under stirring for 12 hours. Odorless, easily available and cheap elemental sulfur is used as a sulfur source, and the method has the advantages of simple and easily available raw materials, simple operation, relatively mild conditions, wide substrate universality, high yield and good functional group compatibility.
Owner:WENZHOU UNIVERSITY
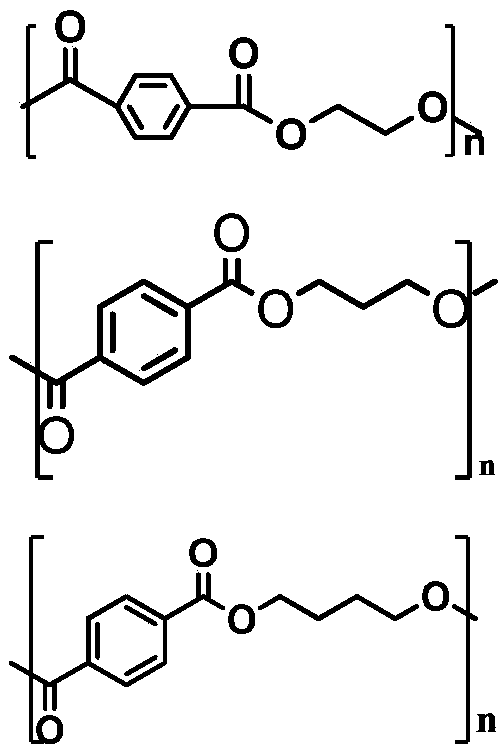
Method for converting PET, PTT and PBT products into cyclo-hydrocarbons in aviation kerosene range
InactiveCN109705985ARaw materials are cheap and easy to getThe synthesis method is simpleFatty acid hydrogenationLiquid hydrocarbon mixture productionPolytetramethylene terephthalatePolyethylene terephthalate glycol
The invention relates to a method for directly converting wasted fibers, thin films and plastic bottles, which are manufactured from polyethylene terephthalate (PET), polytrimethylene terephthalate (PTT) and polybutylene terephthalate (PBT), into cyclo-hydrocarbons in aviation kerosene range. The method can be used for hydrodeoxygenation with a biomass oxygen-containing compound to acquire a fuelwhich contains a chain hydrocarbon, a cyclo-hydrocarbon and an aromatic hydrocarbon, thus solving the defect that a biomass aviation fuel is lack in aromatic hydrocarbons and is poor in volumetric calorific value and sealability. By using the waste wasted fibers, thin films and plastic bottles manufactured from the PET, PTT and PBT, the method is low in cost; the waste wasted fibers, thin films and plastic bottles manufactured from the PET, PTT and PBT, being white garbage, poses a great threat to environment, so that the method not only solves the problem of aviation fuel to certain degree but also solves environmental problems due to the white pollution. The method has potential advantages in future industrial application.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
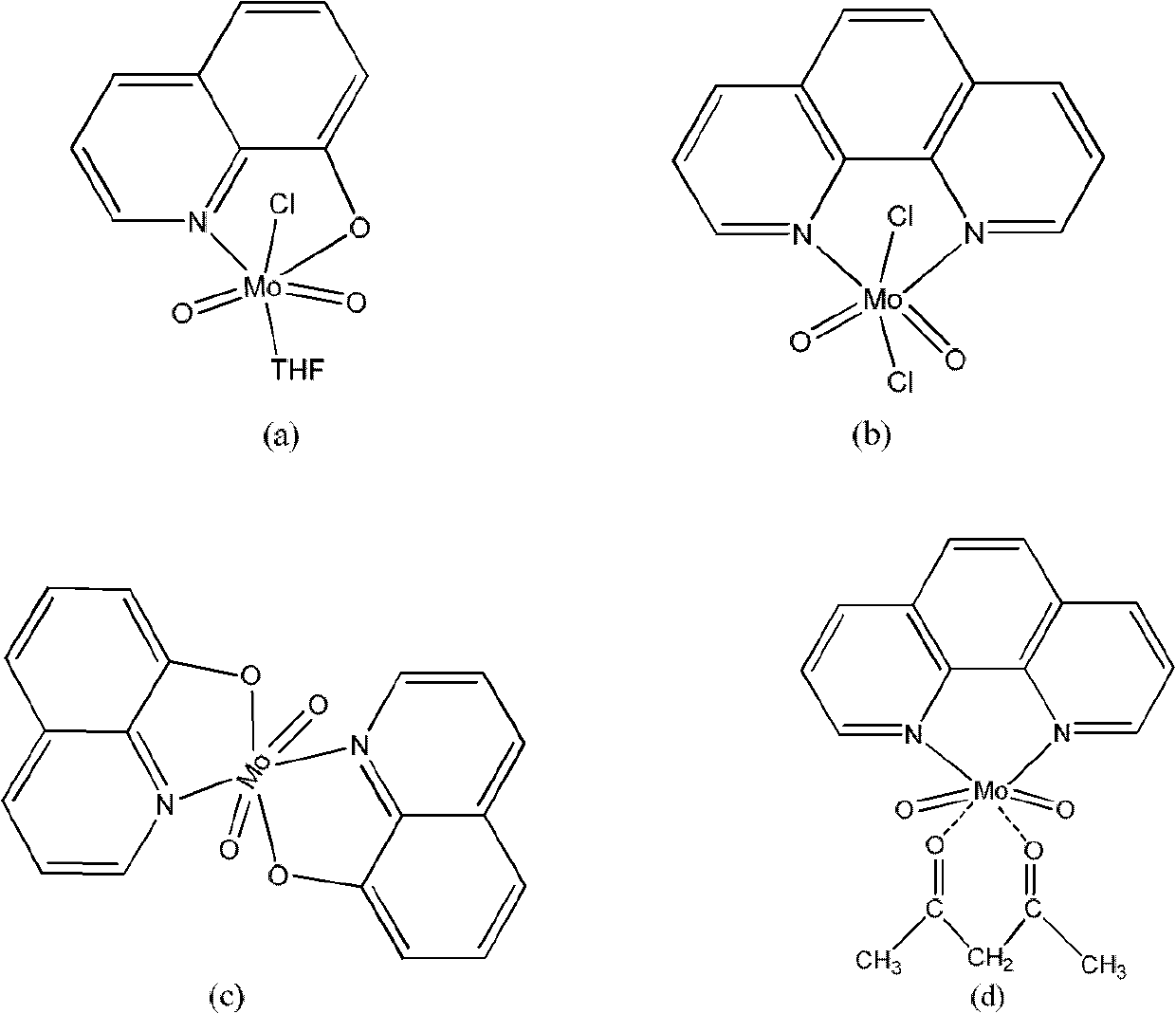
Novel epoxidation catalyst, preparation method and applications
InactiveCN102658203AStable performanceEasy to recycleOrganic-compounds/hydrides/coordination-complexes catalystsGroup 6/16 element organic compoundsAcetylacetoneCoordination complex
The invention discloses a novel epoxidation catalyst, a preparation method and applications. The novel epoxidation catalyst adopts molybdenum dioxide adduct and 8-hydroxyquinoline or 1, 10-phenanthroline as bidentate ligand to synthesize four types of molybdenum (VI) complexes which are monochloro tetrahydrofuran dioxo-molybdenum 8-tumex, 1, 10-phenanthroline dichloro-dioxo molybdenum complex, di (8-tumex) dioxo-molybdenum complex and 1, 10-phenanthroline acetylacetone dioxo molybdenum complex. The structural formulas are shown in the specification. The molybdenum (VI) complexes containing 8-hydroxyquinoline or 1, 10-phenanthroline ligand has the advantages that the properties are stable, the recovery is easy, the synthetic method is simple, the synthetic process is easy to operate, and the epoxidation catalytic effect is good; and the substrate of the epoxidation catalyst is strong in universality, and the epoxidation catalyst is high in activity and good in selectivity, so that the production efficiency of the epoxidation industry is improved, and the cost is reduced.
Owner:HENAN VOCATIONAL COLLEGE OF CHEM TECH
Preparation method of alkyl nitrile compound
ActiveCN111484472AReact efficientlyImplement responseGroup 4/14 element organic compoundsOrganic compound preparationOrganosolvCombinatorial chemistry
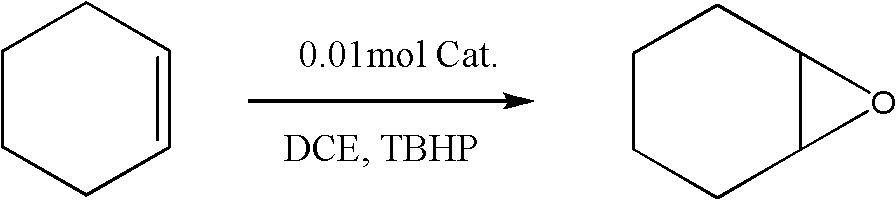
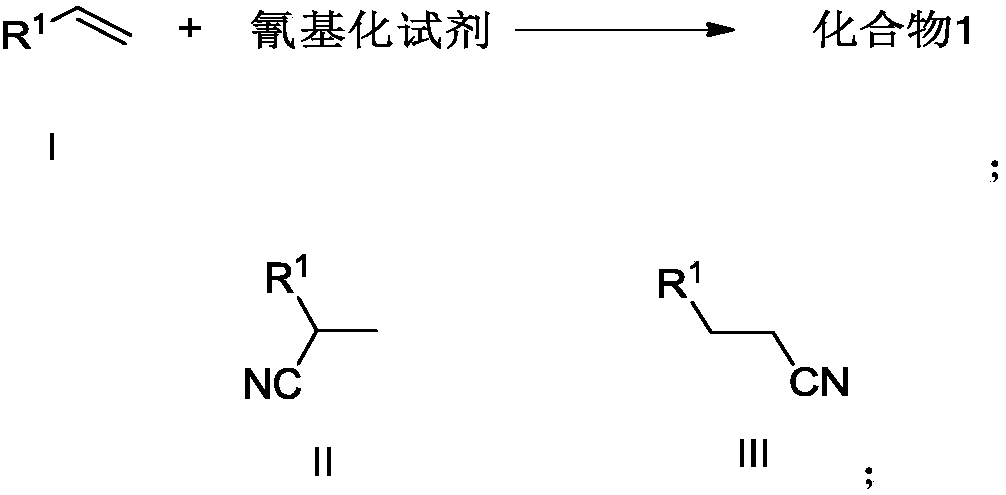
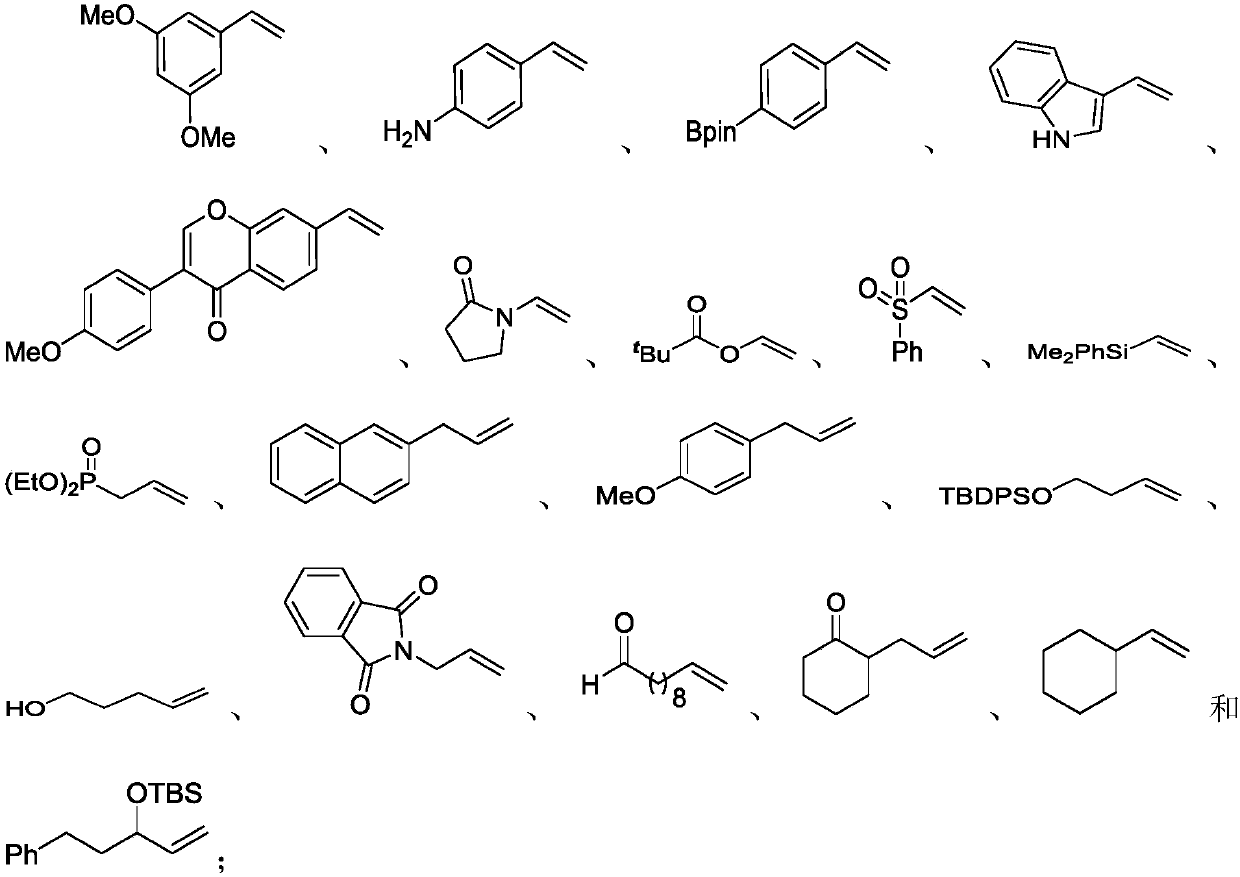
The invention discloses a preparation method of an alkyl nitrile compound. Specifically, the preparation method comprises the following step: in an organic solvent, in the presence of a protective gasand under the action of a catalyst, carrying out a reduction reaction as shown in the specification on olefin as shown in a formula I, a cyanation reagent and water, wherein the alkyl nitrile compound 1 is a compound II and / or a compound III. The preparation method provided by the invention is mild in condition, can realize hydrocyanation of olefin more safely and efficiently, and has good substrate universality and functional group compatibility.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Synthesis method of alpha-keto amide compound
InactiveCN103275027ALow toxicityCheap and accessible environmentSulfonic acid amide preparationSolventToxicity
The invention discloses a synthesis method of an alpha-keto amide compound, which is shown in formula II. According to the synthesis method, an alynyl-amine compound shown in formula I is used as a raw material and then added into a CH3CN / H2O solvent to react so as to obtain the Alpha-keto amide compound shown in formula II under the action of a copper catalyst / Selectfluor oxidant system. The synthesis method provided by the invention has the advantages that the catalyst is low in cost and small in toxicity, the oxygen source is easy to get, environmental protection is realized, the reaction condition is mild, functional groups are good in universality, and the operation is convenient.
Owner:嘉兴南洋万事兴化工有限公司
Difluoroethyl-containing nitrosamine compounds and preparation method thereof
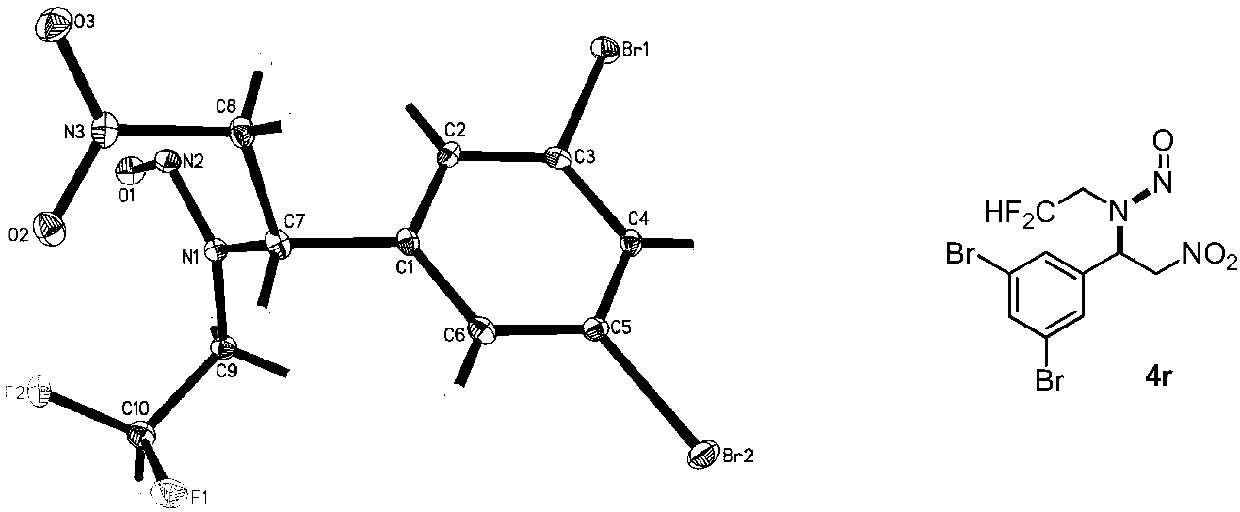
InactiveCN107827774AEasy to derivatizeMild reaction conditionsCarboxylic acid nitrile preparationOrganic compound preparationNitroethyleneCarboxylic acid
The invention discloses difluoroethyl-containing nitrosamine compounds and a preparation method thereof. A series of difluoroethyl-containing nitrosamine derivatives are obtained by taking carboxylicacid as a catalyst and carrying out an aza-Michael addition / nitrosation tandem reaction on three components, i.e., difluoroethylamine, tert-butyl nitrite and 1-aryl 2-nitroethylene at room temperaturein absence of other organic solvents. Molecular skeletons of the compounds contain potentially bioactive difluoroethyl and nitrosamine groups, thus establishing a material basis for drug discovery and druggability evaluation, and having an important application value. The preparation method provided by the invention adopts the easy-to-obtain raw materials, does not need an additional organic solvent, and has the advantages of mild reaction conditions, simple operation, high yield and good substrate universality.
Owner:ZUNYI MEDICAL UNIVERSITY
N-heterocyclic carbene catalytic functionalized imine as novel 1, 4-dipole synthon and synthesis application thereof
ActiveCN112778328ALower synthesis costAchieving Stereodiversity SynthesisOrganic chemistryChemical synthesisAchirality
The invention relates to an N-heterocyclic carbene catalytic functionalized imine as a novel 1, 4-dipole synthon and application thereof, and belongs to the field of chemical synthesis. Under the mild reaction condition, a chiral N-heterocyclic carbene catalyst is used for catalyzing and activating aldimine, a novel aza 1, 4-dipole synthon is obtained under the oxidation condition, and the novel organic synthon can be further subjected to 4 + 2 cyclization reaction with trifluoroacetophenone, isatin and an isatin-derived imine substrate to generate a heterocyclic compound with a novel structure and a chiral quaternary carbon center. According to the method disclosed by the invention, the N-heterocyclic carbene catalyst with the same chiral configuration and an achiral thiourea catalyst are used for co-catalysis, so that three-dimensional diverse synthesis of the trifluoroacetophenone compound can be realized. The method is mild in condition and efficient in reaction and has good substrate universality, and the reported aldimine-derived 1, 4-aza dipole synthon provides an important method for synthesizing various functional nitrogen heterocyclic compounds and has the potential of being applied to industrial production.
Owner:NANJING UNIV OF TECH
Method for synthesizing sulfone compounds under photocatalysis condition
ActiveCN112574077AEfficient synthesisSimple and fast operationOrganic chemistryOrganic compound preparationSulfonateAryl
The invention belongs to the technical field of compound preparation, and particularly relates to a method for synthesizing sulfone compounds under a photocatalysis condition. Aromatic hydrazine and sulfinate are used as raw materials, and under the action of alkali and a solvent, a sulfone compound is generated through reaction under the condition of air or oxygen under the illumination of visible light. According to the method disclosed by the invention, aryl hydrazine is used as an arylation reagent, polyacid salt is used as a catalyst or an organic photosensitizer is used as a catalyst, and the sulfones compound can be efficiently synthesized by coupling with sulfinate under the condition of room temperature through visible light irradiation. The method has good substrate universalityand relatively mild reaction conditions, is not only a substitute for synthesizing sulfone compounds by coupling from simple substrates reported at present, but also broadens the new application of the polyacid salt in the field of photocatalysis.
Owner:HENAN UNIVERSITY
Synthetic method of beta-iodo-alkenyl sulfone compound
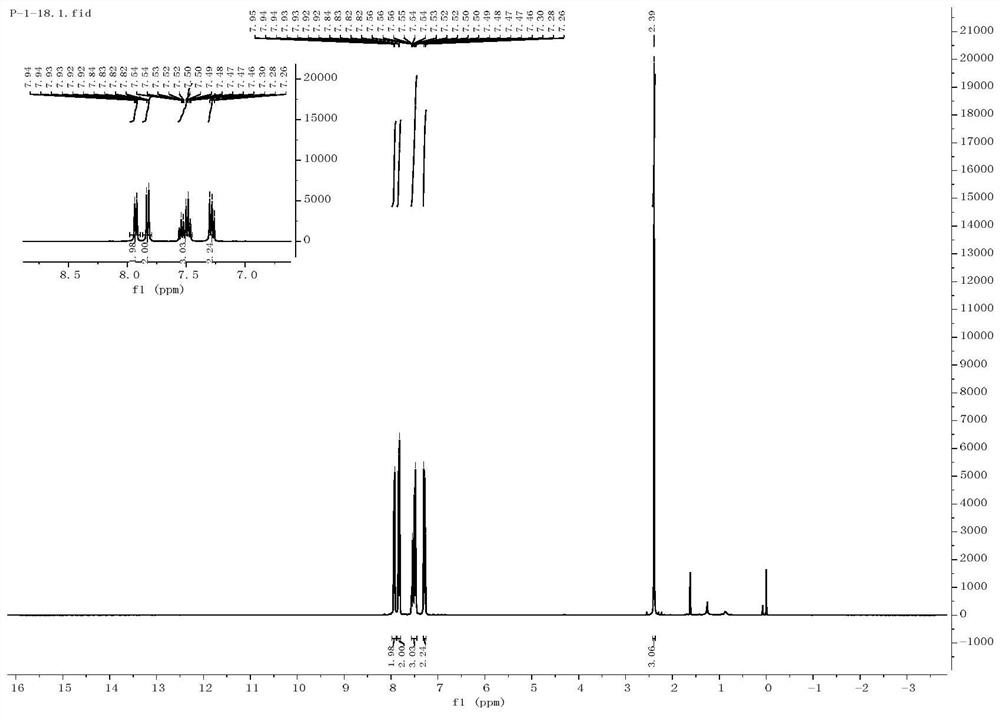
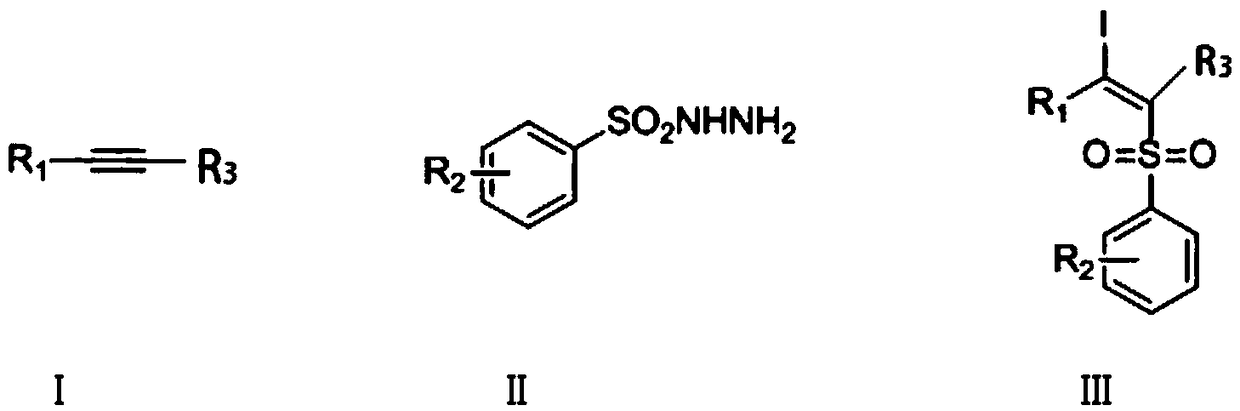
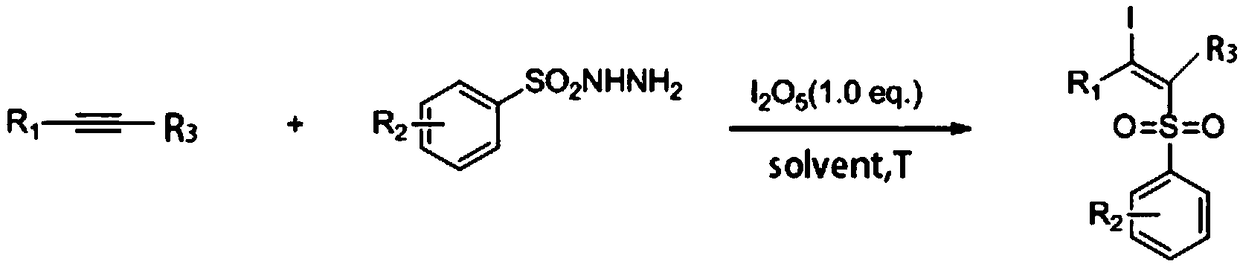
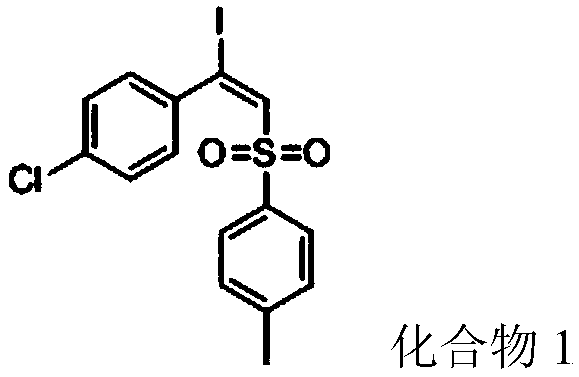
ActiveCN108997178AHigh yieldReduce usageOrganic compound preparationFunctional group formation/introductionSynthesis methodsAlkyne
The invention discloses a synthetic method of a beta-iodo-alkenyl sulfone compound. The synthesis method comprises the following steps: mixing an alkyne derivative, a sulfonyl hydrazine derivative, iodine pentoxide and a solvent to obtain a mixed solution, carrying out stirring for a reaction, carrying out cooling after the reaction is finished, and carrying out column chromatography separation toobtain the beta-iodo-alkenyl sulfone compound represented by a formula III. According to the synthesis method, the iodine pentoxide is used as an iodine source and an initiator, the raw materials arecheap and are simple and easy to obtain, the application range of alkyne and sulfonhydrazide compounds is wide, reaction conditions are mild, post-treatment is simple, and the product yield is high.In addition, use of peroxides is avoided, so that the method is safe and is friendly to the environment, and reaction toxicity is low.
Owner:GUANGZHOU YUEWANG AGRI CO LTD
Visible light induced polyfluoroalkyl aldehyde hydrazone derivative and synthesis method thereof
ActiveCN110183400AGood substrate universalityThe reaction steps are simple to operateOrganic chemistryIodideAldehyde
The invention discloses a visible light induced polyfluoroalkyl aldehyde hydrazone derivative and a synthesis method thereof. According to the polyfluoroalkyl aldehyde hydrazone derivative, an organicdye which is cheap and clean is adopted as a catalyst, and hydrazone and fluoroalkyl iodide are enabled to react with radiation of visible light, so that synthesis of the polyfluoroalkyl aldehyde hydrazone derivative is successfully achieved, and the polyfluoroalkyl aldehyde hydrazone derivative has the advantages of being good in substrate universality, simple in reaction step operation, low incatalyst cost, friendly to the environment, and the like.
Owner:LIAONING UNIVERSITY OF PETROLEUM AND CHEMICAL TECHNOLOGY
Nitration method of quinoxaline substituted alkane
The invention relates to a synthesis method of an organic compound, and provides a nitration method of quinoxaline substituted alkane in order to solve the problems that a conventional nitration method is high in energy consumption, a high-purity nitro-paraffin product is difficult to obtain and the actual application of the high-purity nitro-paraffin product is limited. The nitration method comprises the steps as follows: 2-quinoxaline substituted alkane, a catalyst, a nitration reagent, an oxidant and a reaction solvent are sequentially added in a sealed pressure-resistant container, a mixture is heated and reacts under the pressure of 1 bar-10 bar for 6-72 hours in oil bath at the temperature of 50 DEG C-150 DEG C, and quinoxaline substituted alkane is obtained. With the adoption of the method, the required nitration product is obtained under the relative mild condition through very high selectivity, and the phenomenon of high probability of carbon-carbon bond rupture witha traditional method is avoided.
Owner:临沭县益兴供汽维修服务有限公司
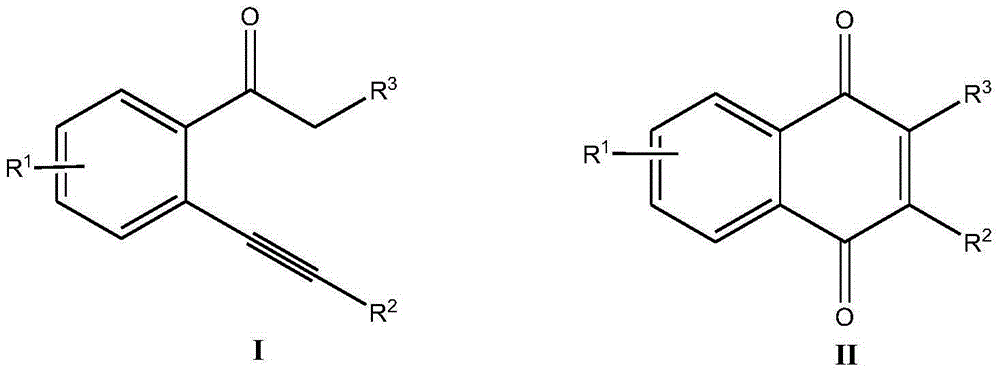
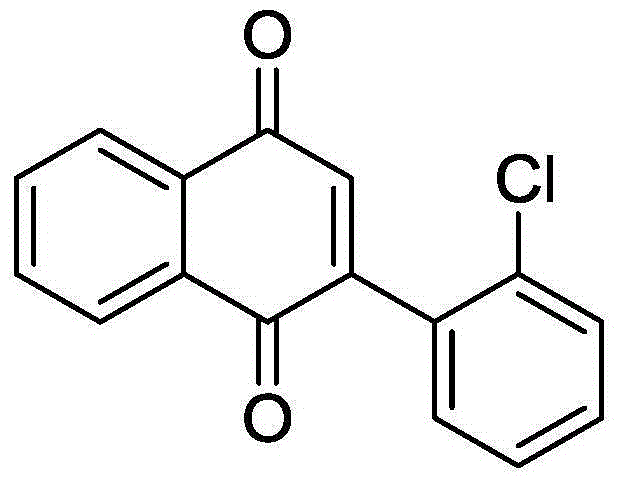
Synthetic method for 2-substituted-1,4-naphthoquinone derivatives
ActiveCN106316817ARaw materials are variableLow costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIodideSolvent
The invention discloses a synthetic method for 2-substituted-1,4-naphthoquinone derivatives. The method comprises the following steps: with 2-alkynylacetophenone compounds as shown in a formula I which is described in the specification as a raw material and dimethyl sulfoxide as an oxidizing agent and a solvent, carrying out a reaction at 80 to 140 DEG C under stirring and the action of a copper catalyst for 2 to 10 h; and subjecting a reaction solution obtained after completion of the reaction to post-treatment so as to obtain the 2-substituted-1,4-naphthoquinone derivatives as shown in a formula II which is described in the specification, wherein the copper catalyst is a mixture of bis(copper trifluoromethanesulfonate) and an inorganic cuprous salt, and the inorganic cuprous salt is one selected from a group consisting of cuprous iodide, cuprous chloride, cuprous bromide and cuprous cyanide. The method has the advantages that the raw materials are variable; the number of the prepared derivatives is great; the catalyst is cheap and easily available; cost is substantially reduced; no pollution is produced; no extra oxidizing agent is needed; dimethyl sulfoxide both functions as the oxygen source and the solvent, so cost is saved; and the method has the characteristics of high reaction yield, good substrate applicability, simple operation, etc.
Owner:ZHEJIANG UNIV OF TECH
Bidentate phosphorus ligand polymer, preparation method thereof and application in olefin hydroformylation reaction
ActiveCN110343209ASave raw materialsThe experiment process is simpleOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsToxicityAldehyde
The invention discloses a bidentate phosphorus ligand polymer represented by a formula (I) shown in the specification, wherein the bidentate phosphorus ligand polymer is formed by copolymerization ofa bidentate phosphorus monomer and styrene. The invention also discloses a preparation method of the bidentate phosphorus ligand polymer, and an application of the bidentate phosphorus ligand polymerin high-selectivity preparation of straight-chain aldehydes in an olefin hydroformylation reaction. In synthesis of the bidentate phosphorus ligand monomer, the used raw materials are cheap, the experimental process is simple, the conditions are relatively mild, reagents used in the experiment are safe and low in toxicity, large-scale production can be further realized, and meanwhile, catalytic activity of the prepared catalyst is improved.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Method for converting polycarbonate compound-containing material into cyclic hydrocarbon in aviation kerosene
InactiveCN111218307AHigh densityRaw materials are cheap and easy to getLiquid carbonaceous fuelsKerosenePolycarbonate
The invention relates to a method for synthesizing cyclic hydrocarbon in aviation kerosene from a polycarbonate compound-containing material. A purpose of the invention is to solve the defect of low density of biomass aviation fuel obtained in the prior art. In the prior art, plastics, plates and glass made of waste polycarbonates are low in cost, and the plastics and the glass made of waste polycarbonates have great threat to the environment as white garbage. According to the invention, the method solves the problem of aviation fuel to a certain extent, solves the environmental problem causedby white pollution, and has potential advantages in future industrial application.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
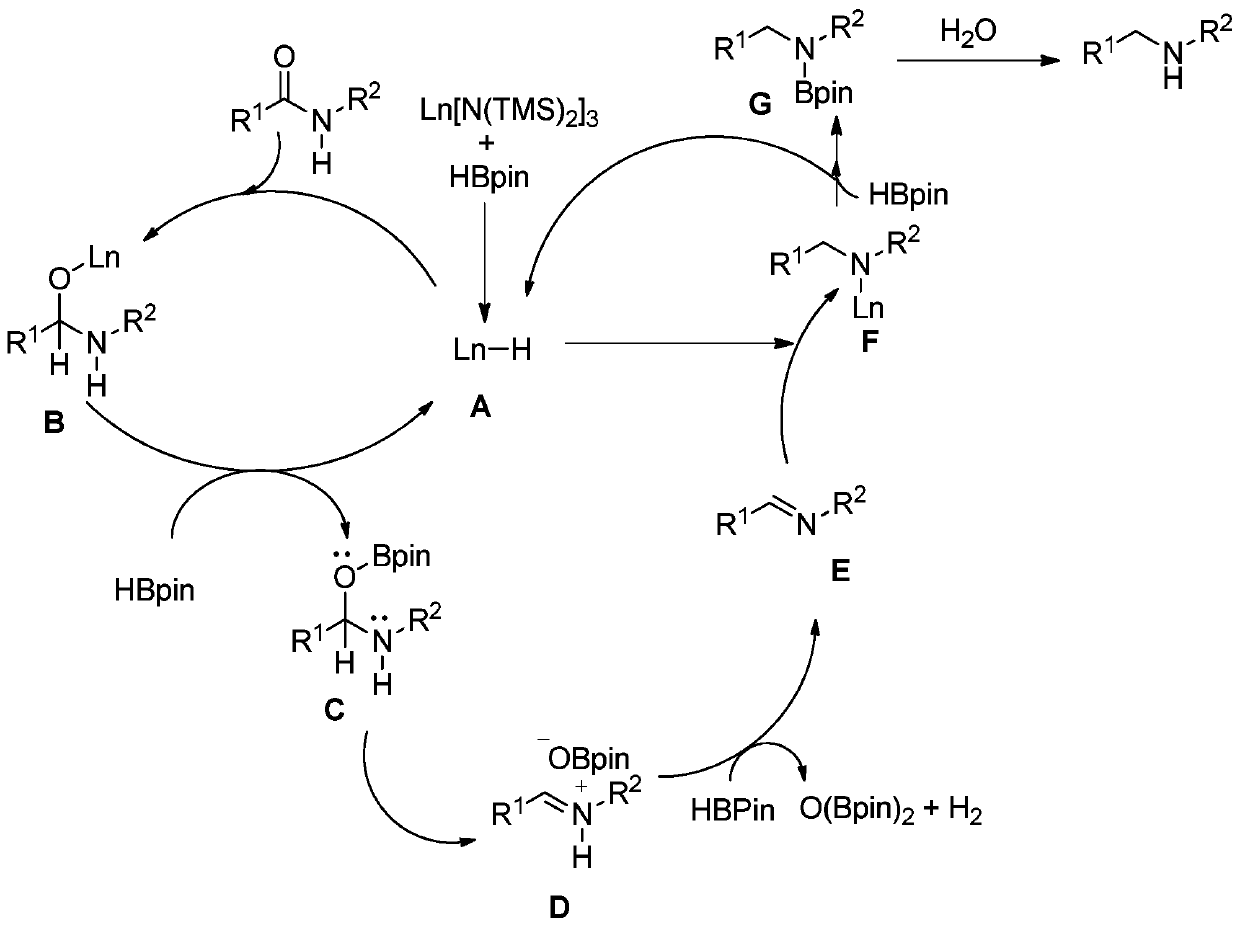
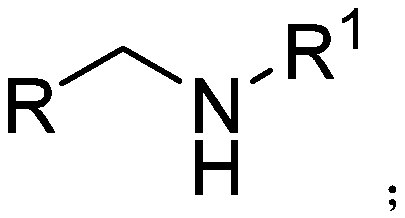
Secondary amine derivative synthesized through rare earth catalysis, and preparation method thereof
ActiveCN110818576AEasy to getWide variety of sourcesAmino preparation from aminesOrganic compound preparationPtru catalystReaction temperature
The invention discloses a secondary amine derivative synthesized through rare earth catalysis, and a preparation method thereof. According to the preparation method, the secondary amine derivative isprepared by carrying out a reaction on reactants of secondary amide and pinacol borane; a rare earth catalyst bis(trimethylsilyl) amino yttrium is added; the reaction temperature is 100-140 DEG C, andthe reaction time is 20-25 h; the whole reaction is carried out under a normal pressure, and the reaction conditions are mild, easy to achieve and safe; the method is simple and convenient to operateand high in reaction selectivity, can directly synthesize the target product without intermediate product separation, can obtain the target product only through a reaction under a normal pressure, issimple in reaction process, has the yield of 90% at most, substantially simplifies the process engineering, reduces the energy consumption, and has high yield; the reaction raw materials are stable and easy to store; a series of secondary amine derivatives can be prepared; and the method has high substrate universality so as to provide the good guarantee for development of related substances related to secondary amine derivatives, and is suitable for large-scale application and popularization.
Owner:WENZHOU UNIVERSITY
Sulfamide benzylation method
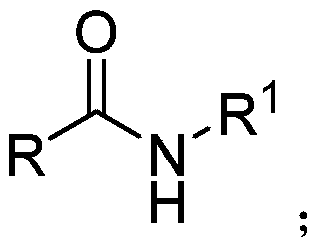
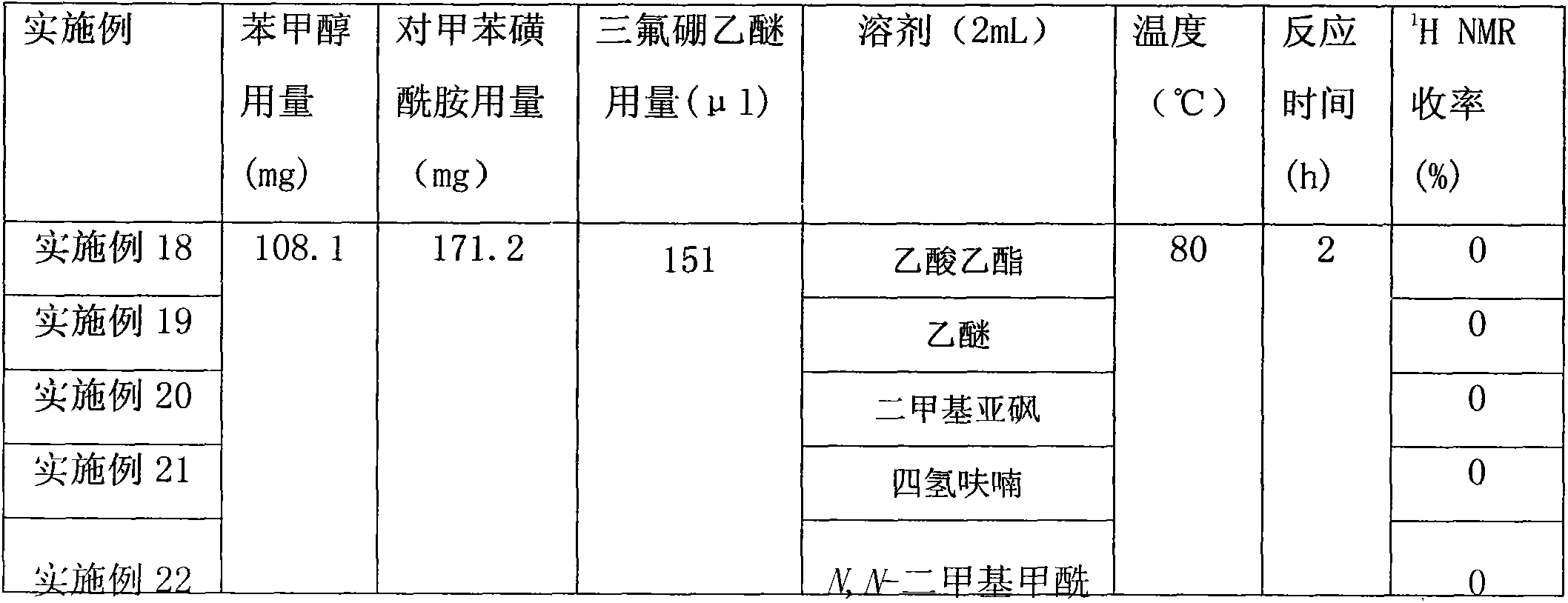
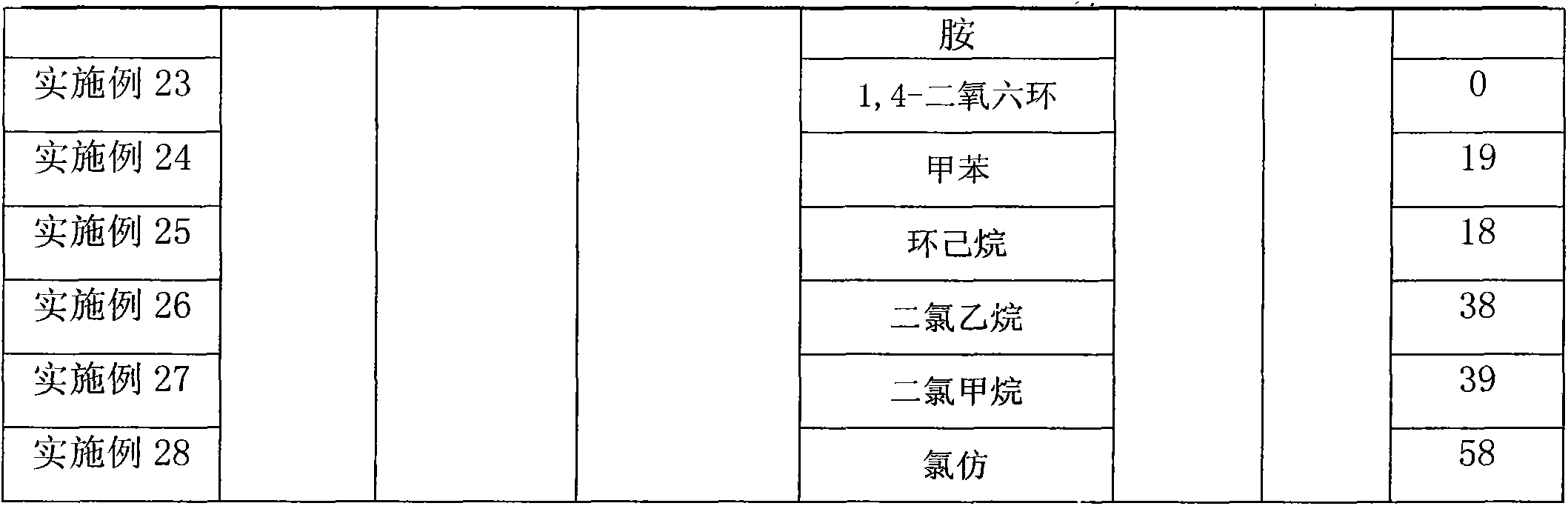
InactiveCN103553978AWide variety of sourcesSimple structureSulfonic acid amide preparationBoron trifluorideSulfamide
The invention relates to a sulfamide benzylation method. According to the sulfamide benzylation method, a finished product is obtained through reaction, concentration and purification by taking various benzyl alcohols and sulfamide as raw materials, boron trifluoride diethyl etherate as a reaction reagent and trichloromethane as a solvent. The sulfamide benzylation method disclosed by the invention has the characteristics of simple method, easiness and convenience for operation, no metal participating reaction, high yield, convenience for application and popularization, and the like, greatly reduces the synthesis cost by taking low-cost benzyl alcohol as a benzylation reagent, can be used for preparing benzyl sulfonamide products with high output rate and multiple varieties and can be widely applied to the industrialized production of benzyl sulfonamides; the product prepared through the sulfamide benzylation method disclosed by the invention can be widely applied to the fields of medicines, pesticides, dyes, engineering plasticizing agents, sterilizing agents, chemical raw materials, and the like and meets the requirements for wide range and large number of the market.
Owner:CHONGQING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com