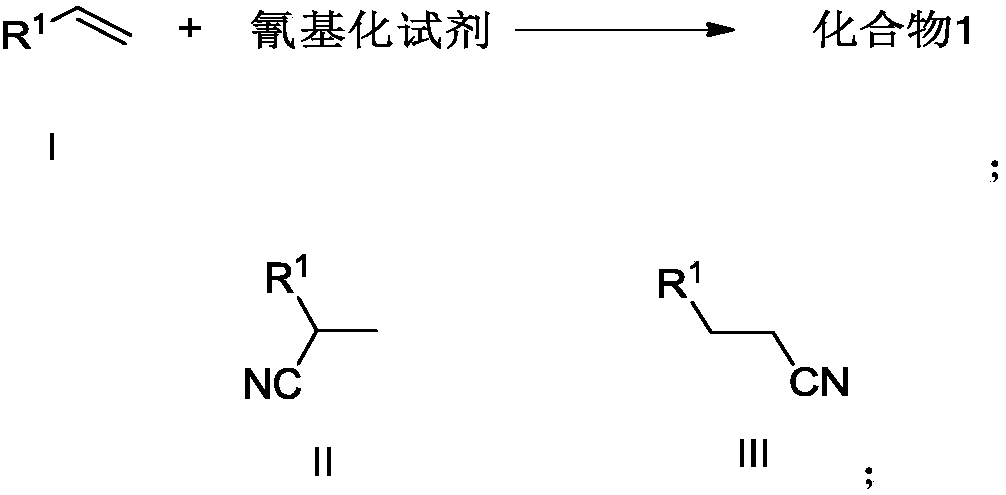
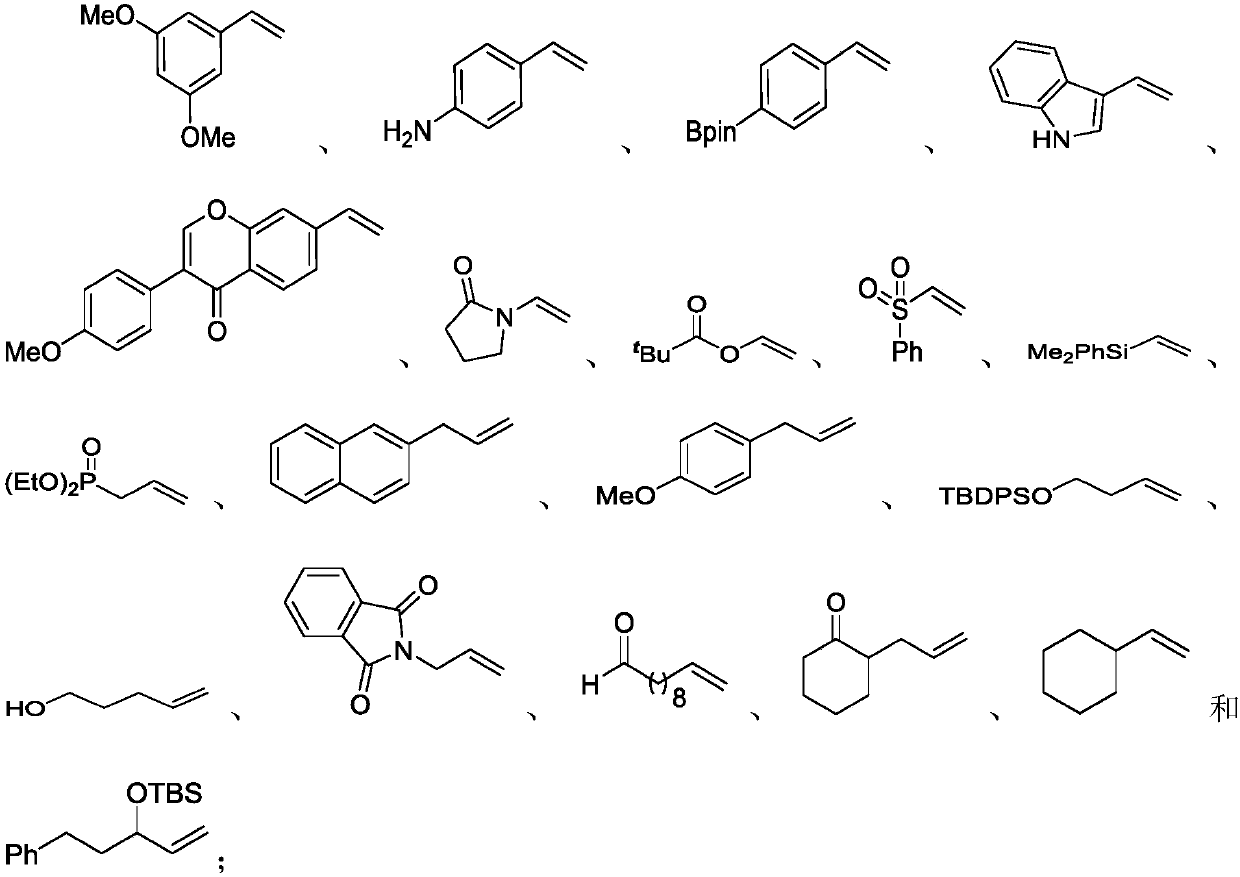
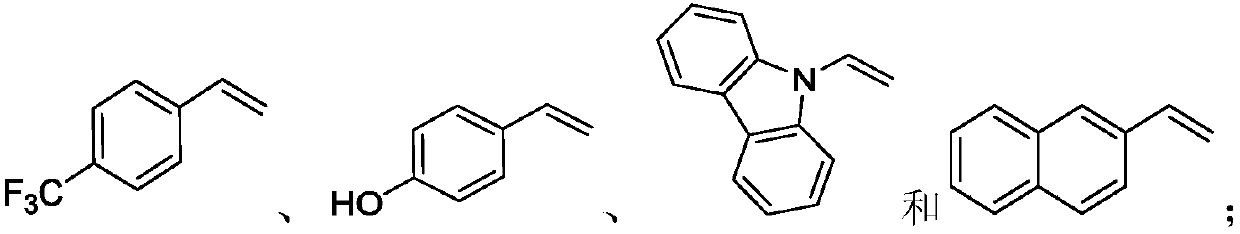
Preparation method of alkyl nitrile compound
A compound, the technology of alkyl nitriles, applied in the field of preparation of alkyl nitriles, can solve the problems of poor compatibility of universal functional groups of substrates, poor efficiency and selectivity of hydrocyanation reaction, and easy volatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0111]
[0112] Under argon protection, methyltriphenylphosphine bromide (2.14g, 6mmol) was dissolved in dry tetrahydrofuran (10ml), and KO was added thereto t Bu (785.5 mg, 7 mmol). The reaction solution was stirred at room temperature for 30 minutes and then cooled to -78°C. A solution of 3,5-dimethoxybenzaldehyde (830.9 mg, 5 mmol) in tetrahydrofuran (5 mL) was added dropwise thereto, and after the addition, the reaction returned to room temperature and continued to stir for 12 h. The reaction was quenched by adding 2 mL of methanol, and the reaction solution was concentrated and purified by silica gel column chromatography. Eluent: petroleum ether / ethyl acetate=10:1, the product is 811mg of colorless liquid, the yield is 99%, 1 H NMR purity greater than 98%. 1 H NMR (400M, CDCl 3 ):δ3.79(s,6H),5.24(d,J=10.8Hz,1H),5.72(dd,J=17.6,0.8Hz,1H),6.38(t,J=2.4Hz,1H),6.56 (d,J=2.4Hz,2H),6.64(dd,J=17.6,10.8Hz,1H). 13 C NMR (100M, CDCl 3 ): δ55.23, 99.97, 104.20, 114.27, 136....
preparation Embodiment 2
[0114]
[0115] Under argon protection, methyltriphenylphosphine bromide (1.7861g, 5mmol) was dissolved in dry tetrahydrofuran (25mL) and cooled to 0°C, and added n BuLi (2 mL, 2.5M in hexane, 5 mmol) and stirring was continued at this temperature for 2 h. A solution of benzothiophene-2-carbaldehyde (811.1 mg, 5 mmol) in tetrahydrofuran (4 mL) was continuously added thereto. The reaction solution was returned to room temperature and continued to stir for 4 h, and then quenched with water. After extraction with ether, wash with water and saturated NaCl, anhydrous Na 2 SO 4 Dry, concentrate by filtration, and purify by silica gel column chromatography. Eluent: petroleum ether / ethyl acetate=10:1, the product is 665mg of white solid, yield 83%, 1 H NMR purity greater than 98%. 1 H NMR (400M, CDCl 3 ):δ5.28(d,J=10.8Hz,1H),5.65(d,J=17.2Hz,1H),6.89(dd,J=17.2,10.8Hz,1H),7.13(s,1H),7.25 -7.31(m,2H),7.65-7.67(m,1H),7.73-7.75(m,1H). 13 C NMR (100M, CDCl 3 ): δ115.89, 122.21, ...
preparation Embodiment 3
[0117]
[0118] Under the protection of argon, formononetin (2.68g, 10mmol) and triethylamine (2.02g, 20mmol) were dissolved in dichloromethane (50mL), and Tf was added thereto after cooling to 0°C 2 O (3.10g, 11mmol), return to room temperature and react for 2h after addition. with saturated NH 4 The reaction was quenched with Cl and extracted with dichloromethane. After combining the organic phases with anhydrous Na 2 SO 4 After drying, filtration and concentration, column chromatography purification, eluent: petroleum ether / dichloromethane=1:2, the intermediate trifluoromethanesulfonyl-protected formononetin was obtained, the product was a white solid 2.76g, the yield 69%, 1 H NMR purity greater than 98%. 1 H NMR (400M, CDCl 3 ):δ3.84(s,3H),6.97(d,J=8.8Hz,2H),7.33(dd,J=8.8,2.4Hz,1H),7.45(d,J=2.0Hz,1H),7.49 (d,J=8.8Hz,2H),8.01(s,1H),8.40(d,J=8.8Hz,1H). 13 C NMR (100M, CDCl 3 ):δ55.26, 111.39, 114.04, 118.47, 118.63 (q, 1 J C-F=321.3Hz), 123.08, 124.14, 125.62, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com