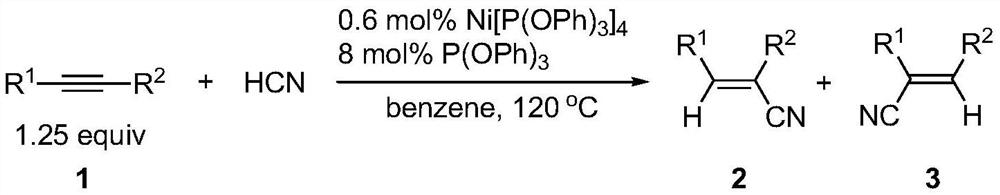
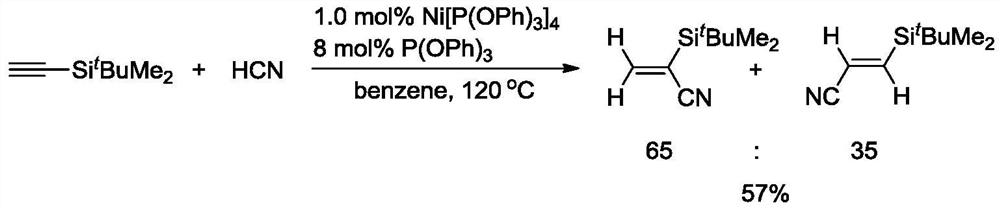
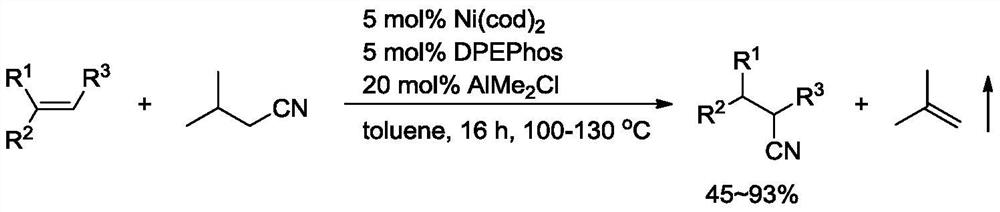
A kind of preparation method of alkenyl cyanide compound
An alkenyl cyanide and compound technology, which is applied in the field of preparation of alkenyl cyanide compounds, can solve the problems of poor stereo and regioselectivity, narrow substrate application range, low efficiency and yield, etc., and achieves simple operation and good functional group compatibility. The effect of the safety of sex, preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1 compound (1a)
[0100]
[0101] In a glove box filled with nitrogen atmosphere, Ni(acac) was sequentially added to the 8.0mL reaction flask 2 (0.025mmol, 6.4mg), Mn (0.1mmol, 5.5mg), Zn(CN) 2 (0.4mmol, 47.0mg), p-methylphenylacetylene (0.5mmol, 58.1mg) and acetonitrile (2.5mL), then the reaction flask was tightly capped and removed from the glove box and water (0.5mL) was added with a syringe, and the Seal the lid of the reaction bottle completely and place it in an oil bath preheated to 25°C for reaction. Stop the reaction after 24 hours. After the reaction liquid is cooled to room temperature, add 10% ammonia water to quench, extract with ethyl acetate, wash with water, Wash with saturated sodium chloride solution, dry over anhydrous sodium sulfate, filter, concentrate, and directly purify by silica gel column chromatography, eluent: petroleum ether to petroleum ether / ethyl acetate = 100:1 gradient elution, the product is a yellow oily liquid 59.1mg, yi...
Embodiment 2
[0102] Example 2 Compound (1a)
[0103] In the embodiment 2-6 of the present invention, the technical scheme is basically the same as that of the embodiment 1, and the only difference is that the ligand is different.
[0104]
[0105] Using the scheme of Example 1, bipyridine was used as the ligand, purified by silica gel column chromatography, eluent: gradient elution from petroleum ether to petroleum ether / ethyl acetate=100:1, the yield was 82%, 1 H NMR purity greater than 98%. 1 H NMR (400MHz, CDCl 3 ): δ2.37(s,3H),6.02(s,1H),6.27(s,1H),7.21(d,J=8.0Hz,2H),7.48(d,J=8.0Hz,2H). 13 C NMR (100MHz, CDCl 3 ): δ21.14, 117.79, 122.63, 125.49, 126.82, 129.45, 129.57, 140.04. HRMS (EI) calculated value C 10 h 9 N[M] + :143.0735, the measured value is 143.0734.
Embodiment 3
[0106] Example 3 Compound (1a)
[0107]
[0108] Using the protocol of Example 1, 4,4'-di-tert-butyl-2,2'-bipyridine is used as a ligand, purified by silica gel column chromatography, eluent: petroleum ether to petroleum ether / ethyl acetate=100:1 Gradient elution, yield 74%, 1 H NMR purity greater than 98%. 1 H NMR (400MHz, CDCl 3 ): δ2.37(s,3H),6.02(s,1H),6.27(s,1H),7.21(d,J=8.0Hz,2H),7.48(d,J=8.0Hz,2H). 13 C NMR (100MHz, CDCl 3 ): δ21.14, 117.79, 122.63, 125.49, 126.82, 129.45, 129.57, 140.04. HRMS (EI) calculated value C 10 h 9 N[M] + :143.0735, the measured value is 143.0734.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com