Method for synthesizing nitryl chloroaniline compound by using micro-channel reactor
A micro-channel reactor, the technology of nitrochloroaniline is applied in the field of nitration of chloroaniline, and achieves the effects of rapid and uniform mixing, maintaining the reaction temperature, and reducing the generation of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) The device used: microchannel reactor, and the heat transfer medium is heat transfer oil.
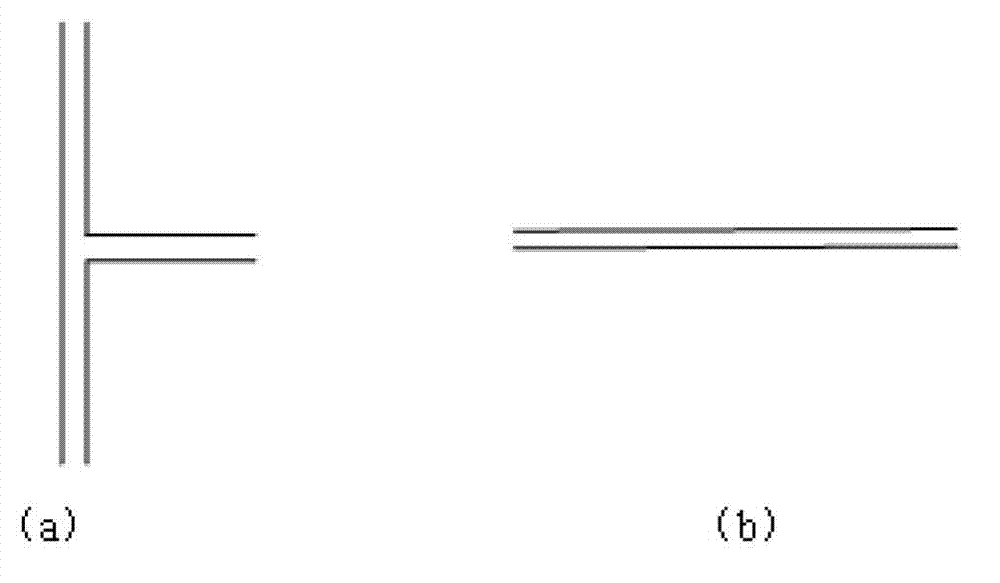
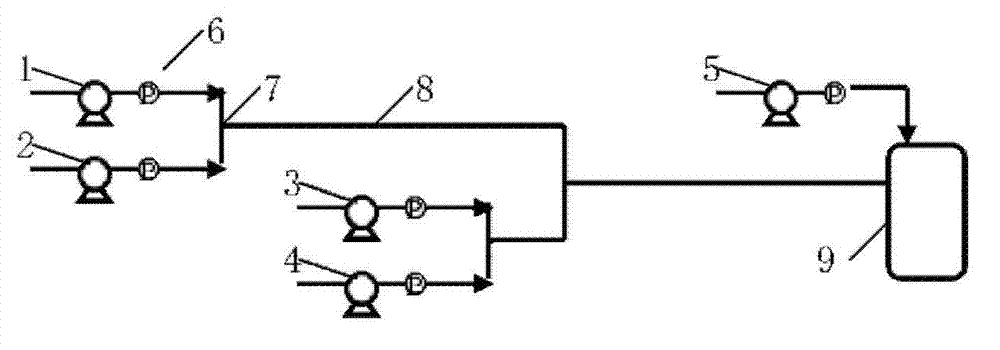
[0034] (2) Refer to image 3Determine the connection mode of the microchannel reactor. The reaction residence time is determined according to the length and flow rate of the straight channel. The circulating heat transfer oil is used as the heat exchange medium, and the reaction temperature is set at 50°C. The molar flow rate ratio of each metering pump is p-chloroaniline:acyl chloride:nitric acid:sulfuric acid=1:1.4:1:0.05, the reaction residence time is 40s, and the reaction product flows out of the reactor and enters the lye hydrolysis tank reactor. The product can be detected by liquid chromatography through extraction, neutralization and washing. At this moment, the conversion rate of p-chloroaniline is 100%, and the mass content of acylated chloroaniline is 5.5% in the product, and the mass content of 5-nitro-p-chloroaniline is 92%.
Embodiment 2
[0036] (1) The device used: microchannel reactor, and the heat exchange medium is water.
[0037] (2) Refer to image 3 Determine the connection mode of the microchannel reactor. The reaction residence time is determined according to the length and flow rate of the straight channel. The circulating heat transfer oil is used as the heat exchange medium, and the reaction temperature is set at 70°C. The molar flow rate ratio of each metering pump is o-chloroaniline: acid anhydride: nitric acid: sulfuric acid = 1: 1.6: 1.1: 0.07, the reaction residence time is 60s, and the reaction product flows out of the reactor and enters the lye hydrolysis tank reactor. The product can be detected by liquid chromatography after extraction, neutralization and washing. At this time, the conversion rate of o-chloroaniline is 100%, the mass content of acylated chloroaniline in the product is 7.5%, and the content of 4-nitro-p-chloroaniline is 64.3% %, the mass content of 3-nitro-p-chloroaniline i...
Embodiment 3
[0039] (1) The device used: microchannel reactor, and the heat exchange medium is water.
[0040] (2) Refer to image 3 Determine the connection mode of the microchannel reactor. The reaction residence time is determined according to the length and flow rate of the straight channel. The circulating heat transfer oil is used as the heat exchange medium, and the reaction temperature is set at 90°C. The molar flow rate ratio of each metering pump is m-chloroaniline: glacial acetic acid: nitric acid: sulfuric acid = 1: 1.8: 1.2: 0.09, the reaction residence time is 80s, and the reaction product flows out of the reactor and enters the lye hydrolysis tank reactor. The product can be detected by liquid chromatography through extraction, neutralization and washing. At this moment, the m-chloroaniline conversion rate is 100%, and the acylated chloroaniline mass content is 2.3% in the product, and the 2-nitro-m-chloroaniline mass content is 20.7%, and the mass content of 4-nitro-m-chlo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com