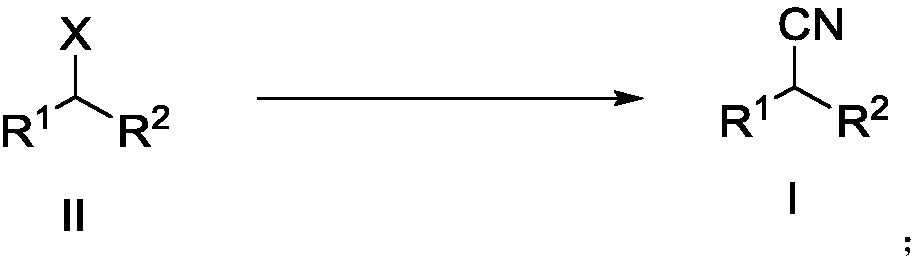
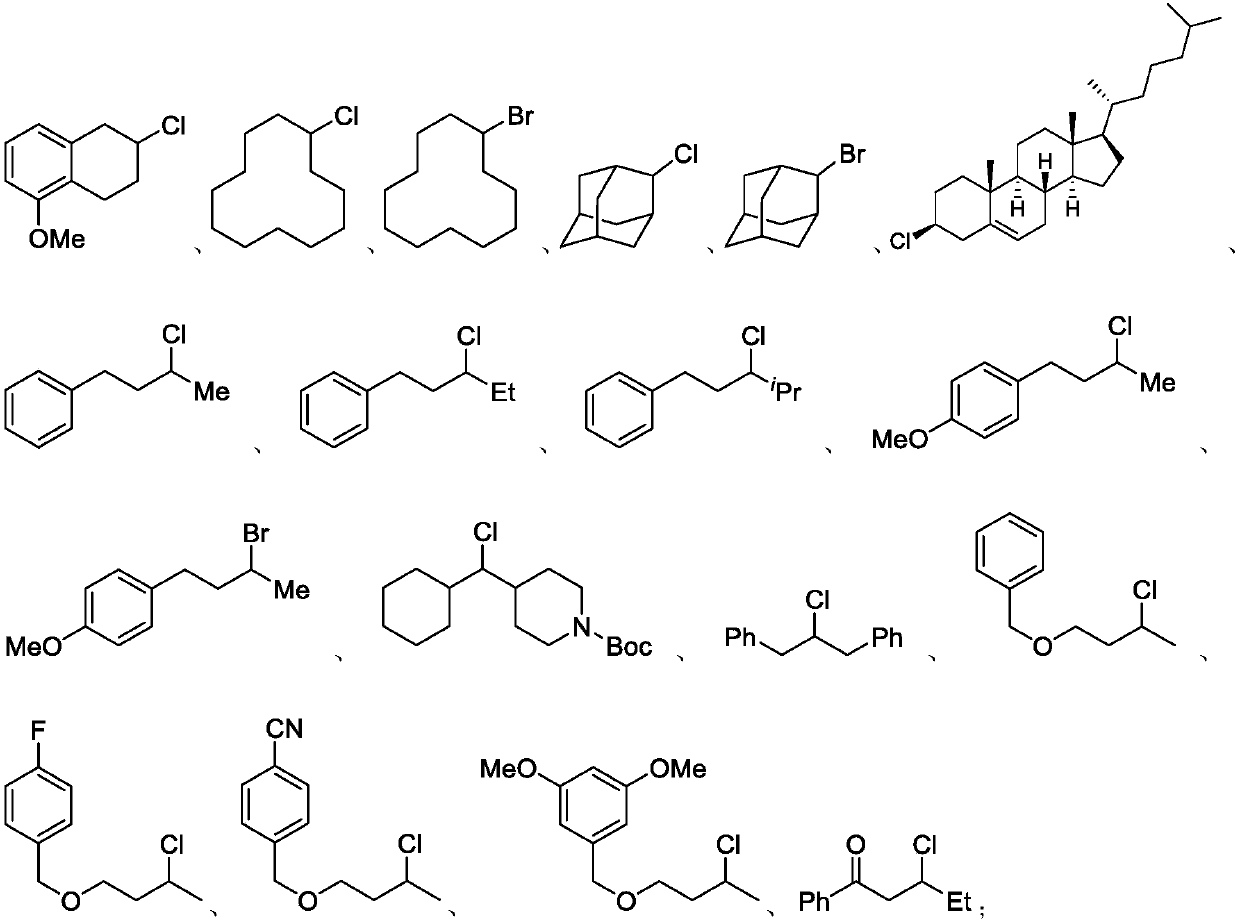
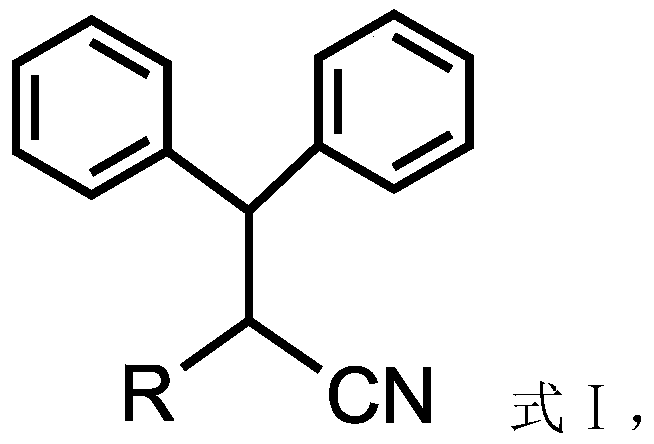
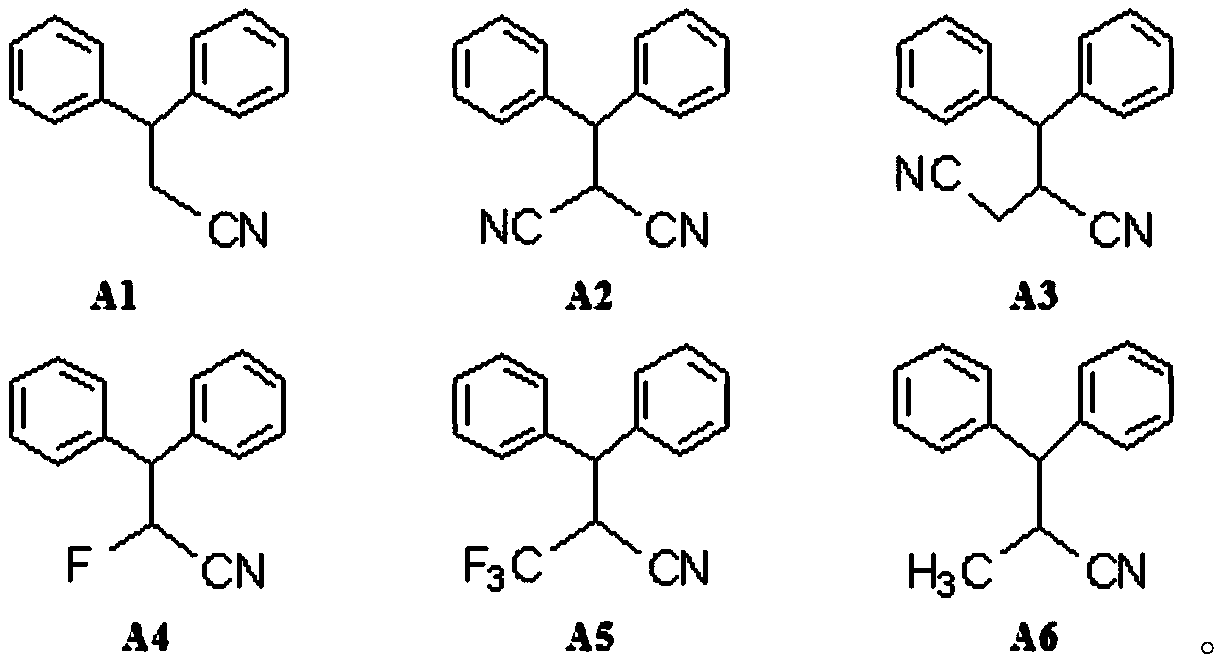
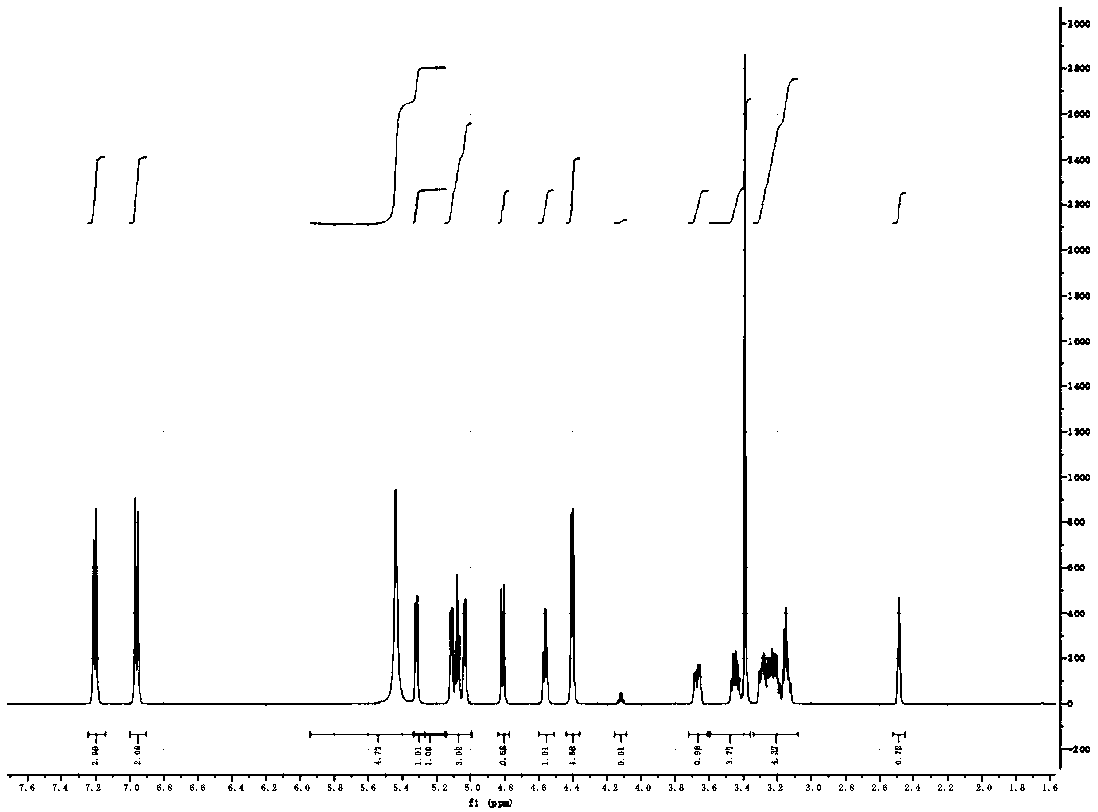
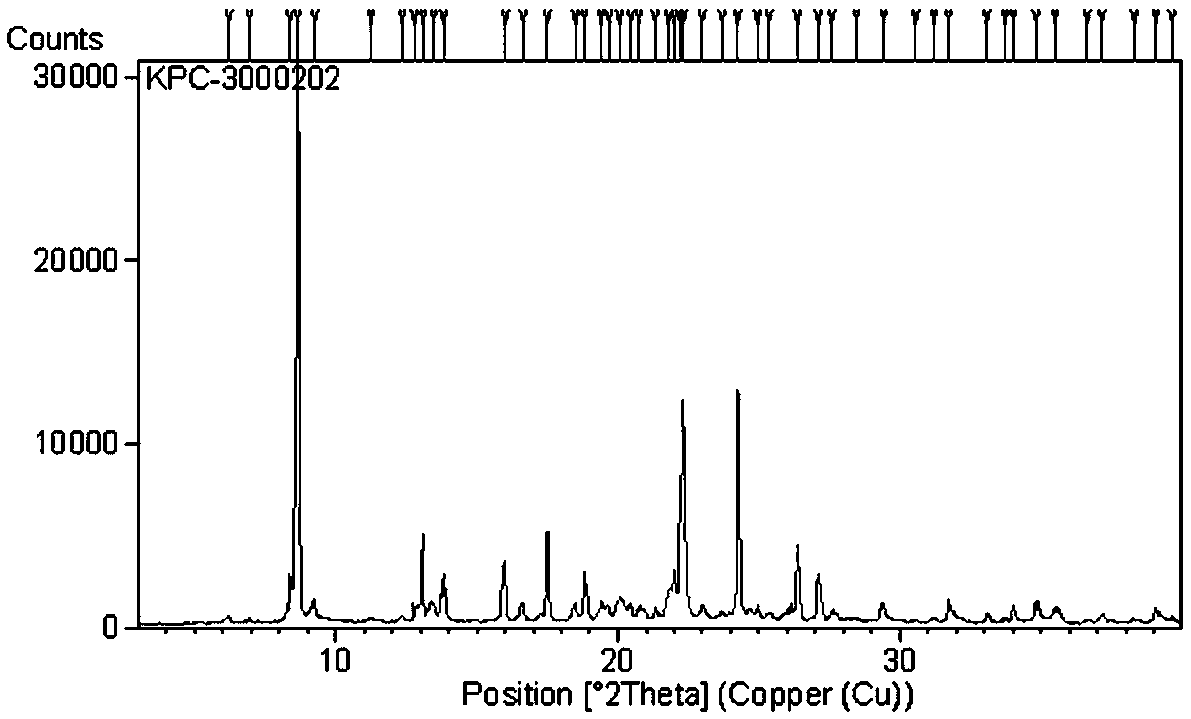
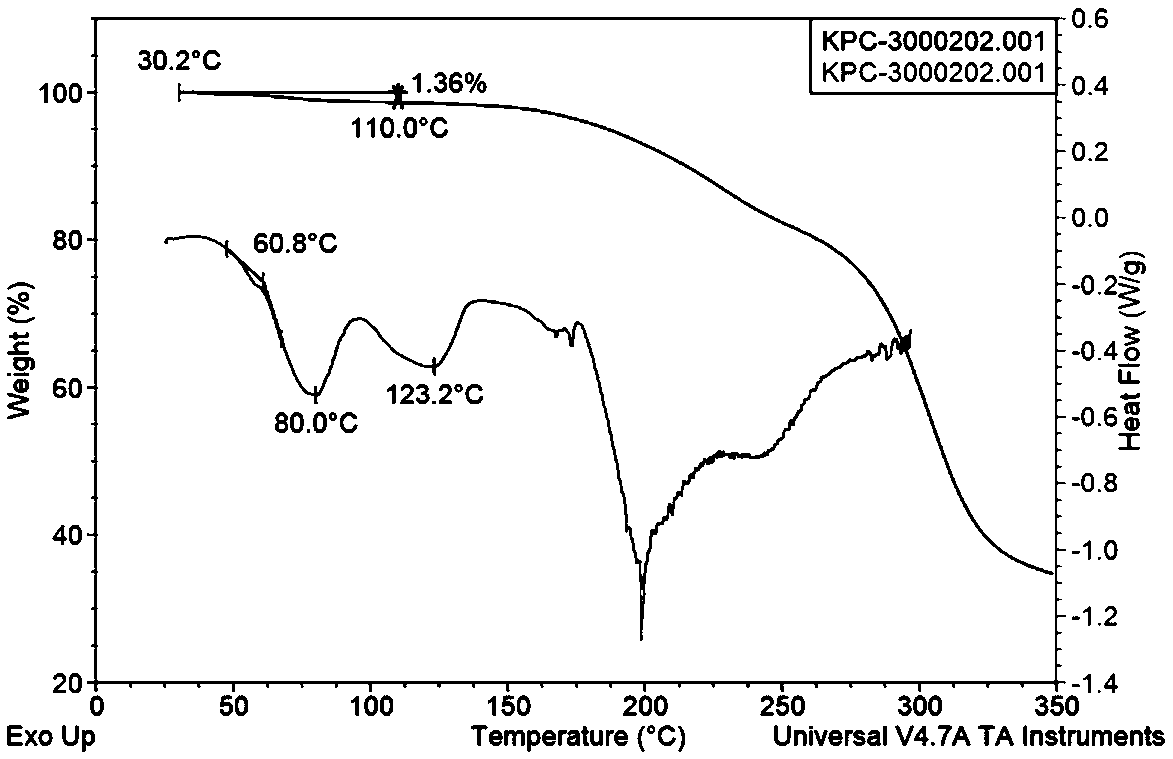
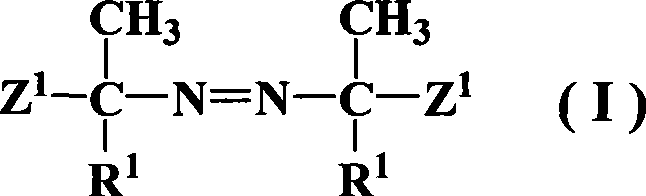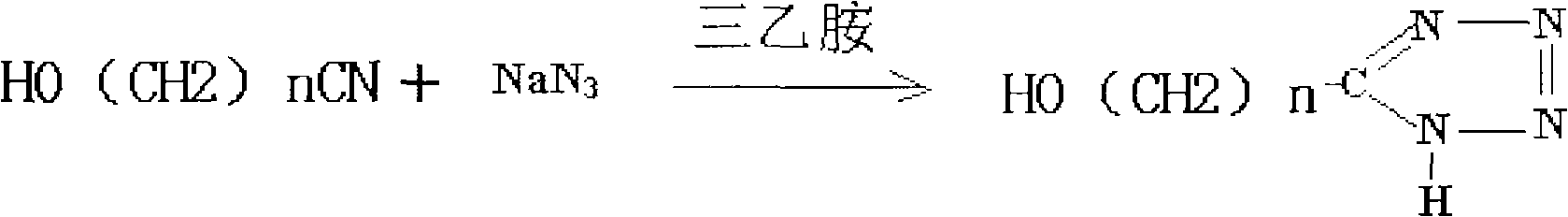Patents
Literature
45 results about "Alkyl nitrile" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alkyl nitriles are sufficiently acidic to form nitrile anions, which alkylate a wide variety of electrophiles. Key to the exceptional nucleophilicity is the small steric demand of the CN unit combined with its inductive stabilization.
Ultraviolet light filter element
InactiveUS6872766B2Increased durabilityAvoid lightLiquid crystal compositionsOther chemical processesThio-Display device
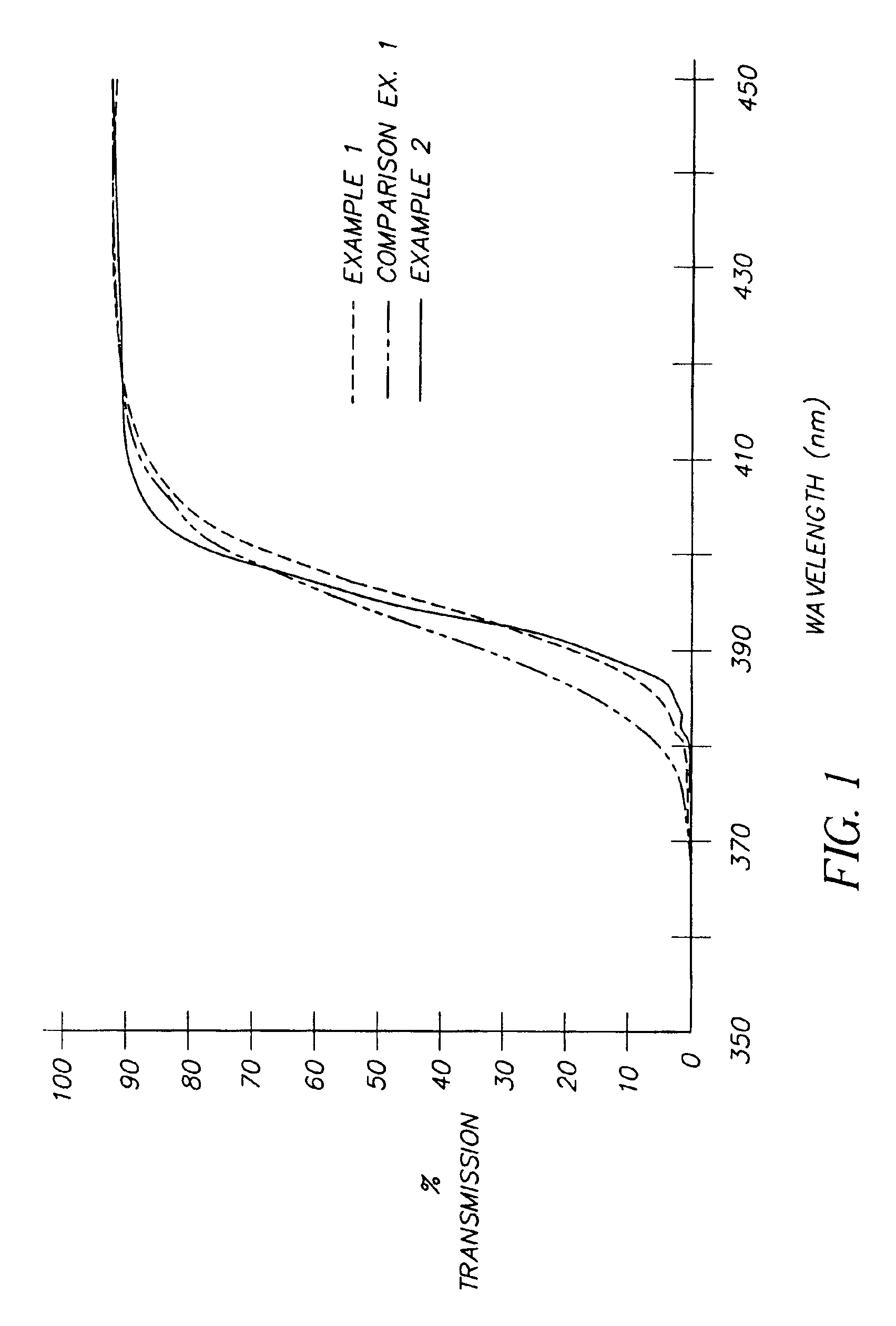
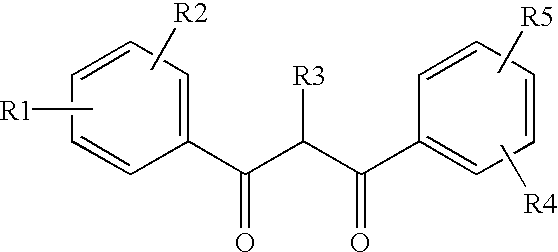
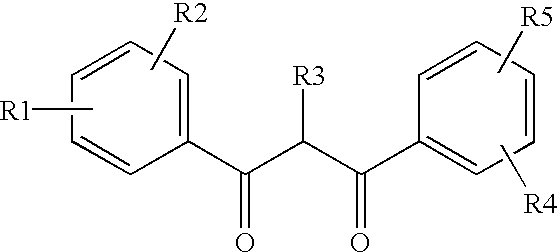
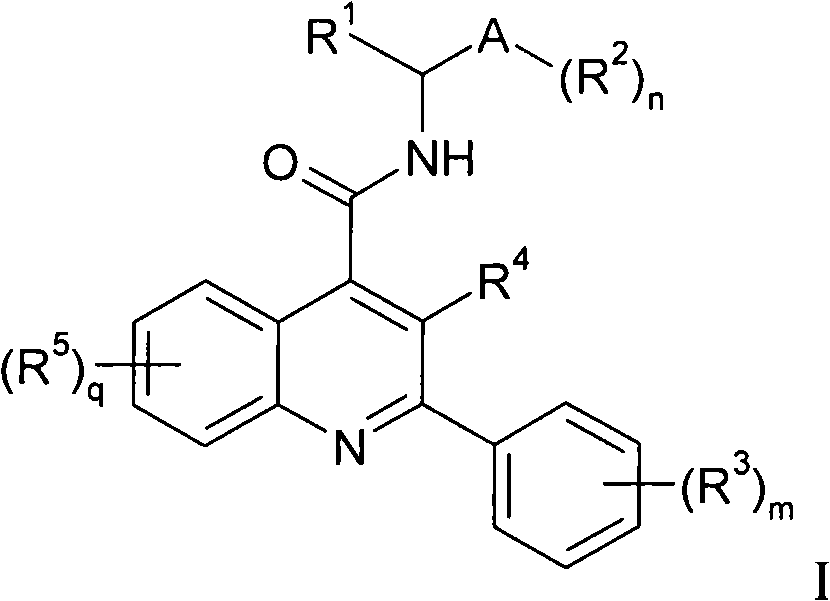
Ultraviolet light absorbing polymer film, coating, or molded article UV filter elements are described which comprise a polymer phase having molecularly dispersed therein a) a first ultraviolet absorbing dibenzoylmethane compound of formula (I) where R1 through R5 are each independently hydrogen, halogen, nitro, or hydroyxl, or further substituted or unsubstituted alkyl, alkenyl, aryl, alkoxy, acyloxy, ester, carboxyl, alkyl thio, aryl thio, alkyl amine, aryl amine, alkyl nitrile, aryl nitrile, arylsulfonyl, or 5-6 member heterocylce ring groups, and b) a second ultraviolet light absorbing compound which absorbs ultraviolet light at a wavelength for which the first compound is deficient at absorbing. In particular embodiments, the second ultraviolet light absorbing compound may comprise a hydroxyphenyl-s-triazine, hydroxyphenylbenzotriazole, formamidine, benzoxazinone, or benzophenone compound. In a specific embodiment of the invention, the above UV absorbing compounds are employed in cellulose acetate film for the fabrication of a protective film for polarizers for use in display applications.
Owner:EASTMAN KODAK CO
Nickelic ternary anode material power lithium ion battery electrolyte and preparation method thereof
InactiveCN109461967AImprove cycle performanceImprove thermal stabilityFinal product manufactureElectrolyte accumulators manufactureHigh temperature storageCarboxylic salt
The invention belongs to the technical field of lithium ion battery preparation, and specifically relates to nickelic ternary anode material power lithium ion battery electrolyte and a preparation method thereof. The electrolyte consists of electrolyte lithium salt, a non-aqueous organic solvent and functional additives, wherein the functional additives comprise an alkyl nitrile additive, fluorinated chain carboxylate, a lithium salt additive and a cathode film forming additive; and the content of the functional additives is 0.5-10% of the weight of the electrolyte. The electrolyte provided bythe invention interacts with transition metal in a nickelic ternary anode material through the functional additives and is decomposed on the surfaces of the anode and cathode to form stable interfacefilms, so as to inhibit the metal ionic catalysis activity and decrease battery side reactions, so that the electrolyte has favorable anti-oxidant and film forming characteristics, is capable of effectively improving the high-temperature storage performance, safety performance and cycle life of nickelic ternary anode material power lithium ion batteries, effectively inhibiting the generation of battery expansion and ensuring the high power characteristic of the batteries at the same time.
Owner:JIANGXI YOULI NEW MATERIALS
Azos thermal initiator, synthetic method and application thereof
InactiveCN101381421AHigh grafting rateTrigger conditions are simpleOrganic chemistryPolyelectrolyte brushesIn situ polymerization
The invention relates to an azo thermal initiator for use in preparation of a nano-sized spherical polyelectrolyte brush, a synthesis method and an application thereof. Firstly, azodiiso-alkylnitrile and diol are taken as raw materials to synthesize an intermediate azodiisoacid diol ester; and then the intermediate reacts with alkyl acrylyl chloride to obtain the novel azo thermal initiator with a C=C double bond as a terminal group. A novel azo thermal initiator both ends of which contain C=C double bonds can be obtained through the separation by a certain method. The thermal initiator can initiate the in-situ polymerization of monomers such as acrylic acid, 4-ethenyl-benzenesulfonic acid sodium salt and so on, on the surfaces of polystyrene milk globules to prepare the nano-sized spherical polyelectrolyte brush with controllable polyelectrolyte length and grafting density.
Owner:EAST CHINA UNIV OF SCI & TECH
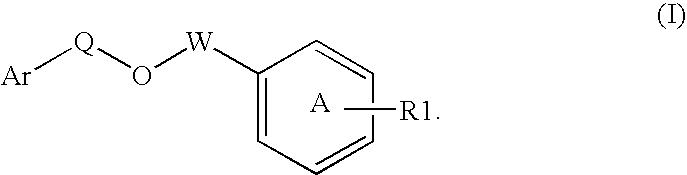
Modulators of peroxisome proliferator activated receptors
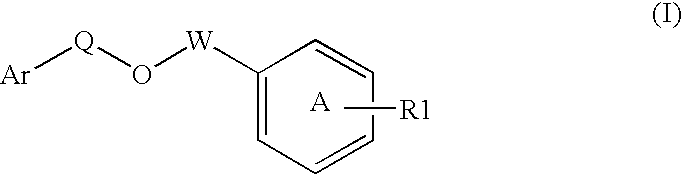
Disclosed is a compound represented by Structural Formula (I): Ar is a substituted or unsubstituted aromatic group. Q is a covalent bond, —CH2— or —CH2CH2—; W is a substituted or unsubstituted alkylene or a substituted or unsubstituted heteroalkylene linking group from two to ten atoms in length, preferably from two to seven atoms in length. Phenyl Ring A is optionally substituted with up to four substituents in addition to R1 and W, R1 is (CH2)n—CH(OR2)—(CH2)mE1, —(CH)═C(OR2)—(CH2)mE, —(CH2)n—CH(Y)—(CH2)mE or (CH)═C(Y)—(CH2)mE; wherein E is COOR3, C1–C3 alkylnitrile, carboxamide, sulfonamide, acylsulfonamide or tetrazole and wherein sulfonamide, acylsulfonamide and tetrazole are optionally substituted with one or more substituents independently selected from: C1–C6 alkyl, haloalkyl and aryl-C0-4-alkyl; R2 is H, an aliphatic group, a substituted aliphatic group, haloalkyl, an aromatic group, a substituted aromatic group, —COR4, —COOR4, —CONR5R6, —C(S)R4, —C(S)OR4 or C(S)NR5R6, R3 is H, an aliphatic group, a substituted aliphatic group, an aromatic group or a substituted aromatic group. Y is O—, CH2—, —CH2CH2— or CH═CH— and is bonded to a carbon atom in Phenyl Ring A that is ortho to R1. R4–R6 are independently H, an aliphatic group, a substituted aliphatic group, an aromatic group or a substituted aromatic group. n and m are independently 0, 1 or 2
Owner:ELI LILLY & CO +1
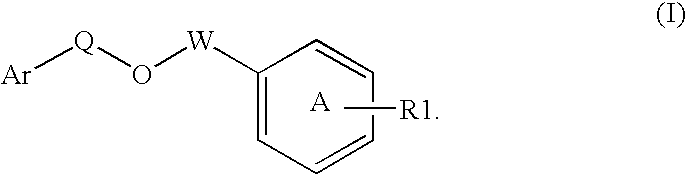
Modulators of peroxisome proliferator activated receptors
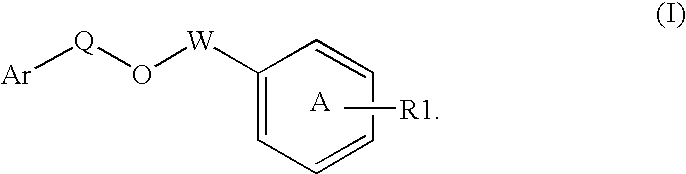
Disclosed is a compound represented by Structural Formula (I): Ar is a substituted or unsubstituted aromatic group. Q is a covalent bond, —CH2— or —CH2CH2—; W is a substituted or unsubstituted alkylene or a substituted or unsubstituted heteroalkylene linking group from two to ten atoms in length, preferably from two to seven atoms in length. Phenyl Ring A is optionally substituted with up to four substituents in addition to R1 and W, R2 is (CH2)n—CH(OR2)—(CH2)nE, —(CH)═C(OR2)—(CH2)nE, —(CH2)n—CH(Y)—(CH2)mE or (CH)═C(Y)(CH2)mE; wherein E is COOR3, C1-C3 alkylnitrile, carboxamide, sulfonamide, acylsulfonamide or tetrazole and wherein sulfonamide, acylsulfonamide and tetrazole are optionally substituted with one or more substituents independently selected from: C1-C6 alkyl, haloalkyl and aryl-Co-4-alkyl; R2 is H, an aliphatic group, a substituted aliphatic group, haloalkyl, an aromatic group, a substituted aromatic group, —COR4, —COOR4, —CONR5R6, —C(S)R4, —C(S)OR4 or C(S)NR5R6, R3 is H, an aliphatic group, a substituted aliphatic group, an aromatic group or a substituted aromatic group. Y is O—, CH2—, CH2CH2— or CH═CH— and is bonded to a carbon atom in Phenyl Ring A that is ortho to R1. R4-R6 are independently H, an aliphatic group, a substituted aliphatic group, an aromatic group or a substituted aromatic group. n and m are independently 0, 1 or 2.
Owner:ELI LILLY & CO +1
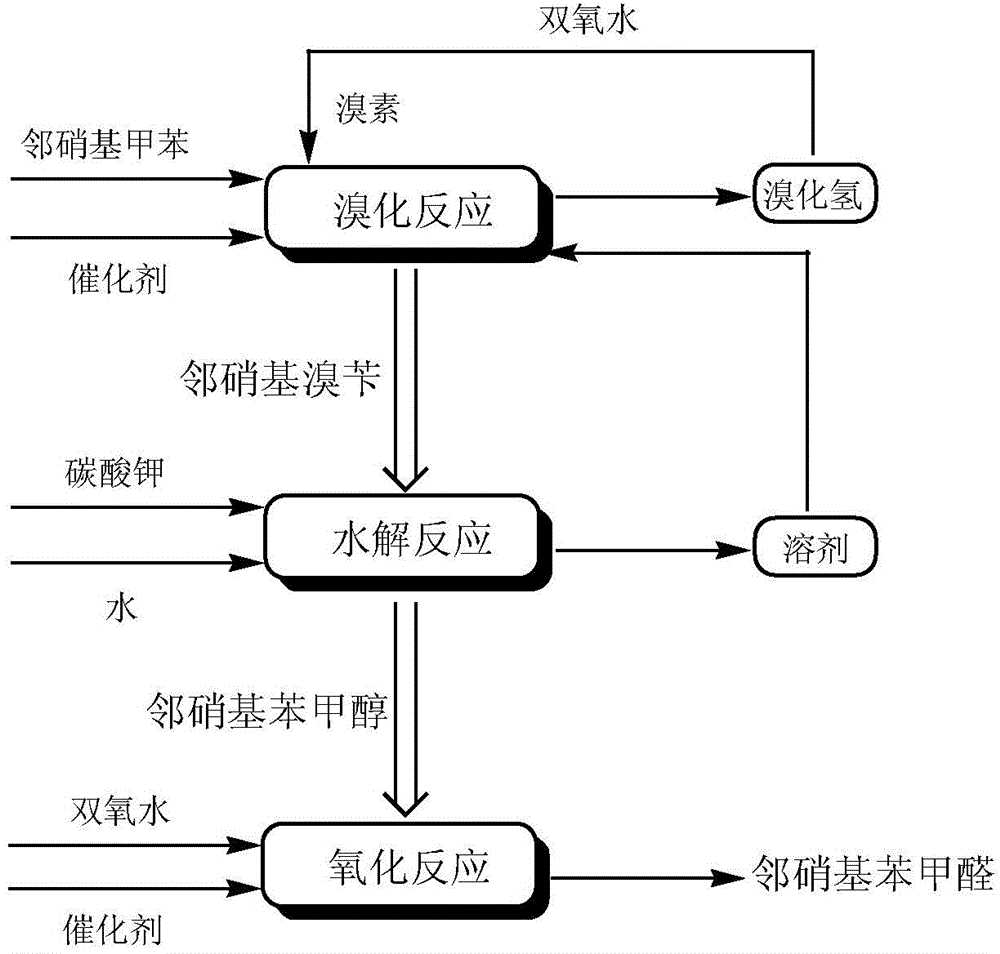
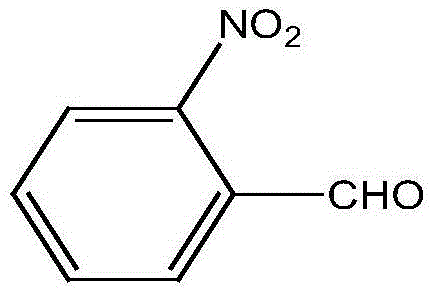
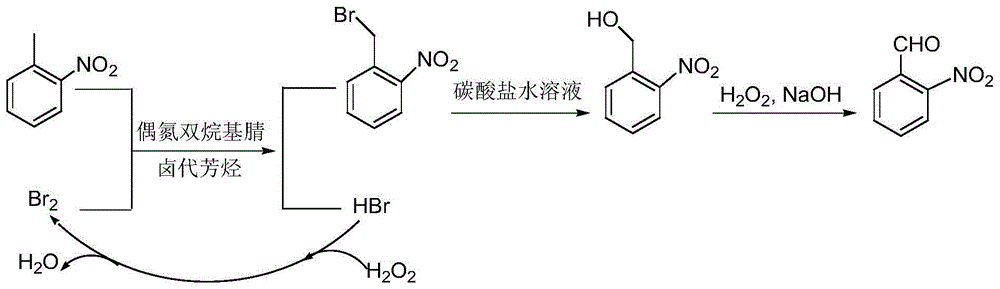
A preparing method of 2-nitrobenzaldehyde
InactiveCN105439867AEasy to cleanReduce pollutionOrganic chemistryOrganic compound preparationCatalytic functionOperation safety
The invention discloses a preparing method of 2-nitrobenzaldehyde. 2-nitrotoluene is adopted as a raw material and is brominated with bromine under catalytic function of azo-bis alkyl nitrile to generate 2-nitrobenzyl bromide and hydrogen bromide. The 2-nitrobenzyl bromide is hydrolyzed under catalytic function of an aqueous carbonate solution to generate 2-nitrobenzyl alcohol. The 2-nitrobenzyl alcohol is oxidized with hydrogen peroxide under catalytic function of sodium hydroxide to generate the objective compound, namely the 2-nitrobenzaldehyde. A hydrogen peroxide oxidation manner is adopted by the method, thus improving cleanliness of industrial preparation reactions, and reducing environment pollution. Oxidation is catalyzed by adopting the inorganic solid alkali catalyst and no metal organic complex catalyst is used, thus improving reaction stability and greatly reducing the cost of industrial preparation. An azo-bis alkyl nitrile solid catalyst in place of a peroxydicarbonate liquid catalyst is adopted to catalyze the bromination, thus improving reaction operation safety of industrial preparation. The method increases the product yield. The yield of the method is increased by about 5% than that of traditional industrial methods at present. The total yield can reach 77% and product purity is higher than 99%.
Owner:NANJING UNIV OF SCI & TECH
Preparation of aromatic nitrile compound by waste-free circulation method
InactiveCN101081821AReduce consumptionFull recoveryCarboxylic acid nitrile preparationOrganic compound preparationChemical industryCyclic process
The present invention is no-waste liquid cyclic process for preparing aromatic amino nitrile compound. The cyclic process synthesizes aromatic amino nitrile compound R1-NH-R2-CN by using aromatic amine compound R1-NH2 and hydroxyl alkyl nitrile compound HO-R2-CN as materials and in several combined cyclic systems, and has no waste liquid drainage. The cyclic process has full utilization of materials, saving in resource, high product yield, zero waste liquid drainage and other advantages, and is suitable for use in chemical industry.
Owner:成都圣洁环保有限责任公司
Flame retardant anti-bacterial composite modifier used in fiber field and preparation method thereof
InactiveCN102619086AAntibacterialFlame retardantGroup 5/15 element organic compoundsFibre treatmentFiberPhosphoric Acid Esters
The invention relates to a flame retardant anti-bacterial composite modifier used in the fiber field and a preparation method thereof. The active components of the flame retardant anti-bacterial modifier are alkyl phosphate and alkyl guanidine; and the preparation method of the flame retardant anti-bacterial composite modifier comprises the following steps of: (1) preparing alkyl tetrol phosphoric acid ester; and (2) taking alkyl tetrol phosphate ester B, alkyl guanidine C and p-benzoic acid alkyl nitrile D which have the equal amount of substance, adding concentrated sulfuric acid and dicyclohexylcarbodiimide / N, N'-diisopropyl carbodiimide (DCC / DIC) as catalysts, wherein the mass ratio of the concentrated sulfuric acid to the alkyl tetrol phosphate ester B is 1:2-4, the mass ratio of DCC / DIC to the alkyl guanidine C is 1:2-5, rising the temperature to 70 to 200 DEG C, after reacting for 3-10h, cooling, filtering and drying to obtain the solid composite modifier E. The flame retardant anti-bacterial composite modifier has the advantages of having the flame retardant anti-bacterial performance, good thermal stability, being capable of meeting the high temperature processing requirement and being not easy to degrade; and products machined and prepared by using the composite modifier are not easy to oxidize and change color; and the flame retardant anti-bacterial composite modifier is widely applied.
Owner:ZHEJIANG JIABAO NEW FIBER GROUP
Preparing and dispersing surface-modified colour pigments
InactiveUS8313576B2Minimal health and environmental impactQuality improvementOrganic chemistryDisazo dyesChlorosulfuric acidPigment
A method of preparing a pigment includes the steps of: a) providing a mixture including:a pigment selected from the group of pigments including at least 50 wt % of C.I. Pigment Violet 23, C.I. Pigment Red 146, C.I. Pigment Red 176, C.I. Pigment Red 177, C.I. Pigment Violet 19 and C.I. Pigment Orange 13 based on the total weight of the pigment;alkyl nitrile; andat least one acid selected from the group consisting of sulfuric acid and chlorosulfuric acid, with the acid present in the mixture in amount of more than 2 wt % based on the total weight of the pigment;b) heating the mixture for more than 2 hours to a temperature of at least 70° C.; andc) filtering the mixture and washing the filtrand with a washing liquid containing water until the filtrate has a pH between 4 and 7. Pigments obtainable by the method and non aqueous pigment dispersions are also disclosed.
Owner:AGFA NV
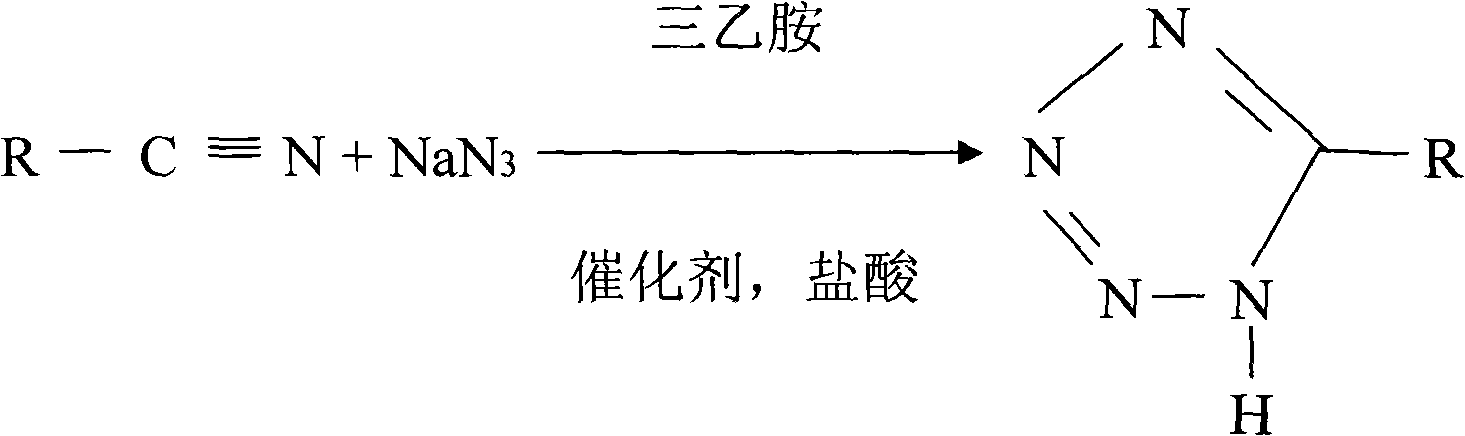
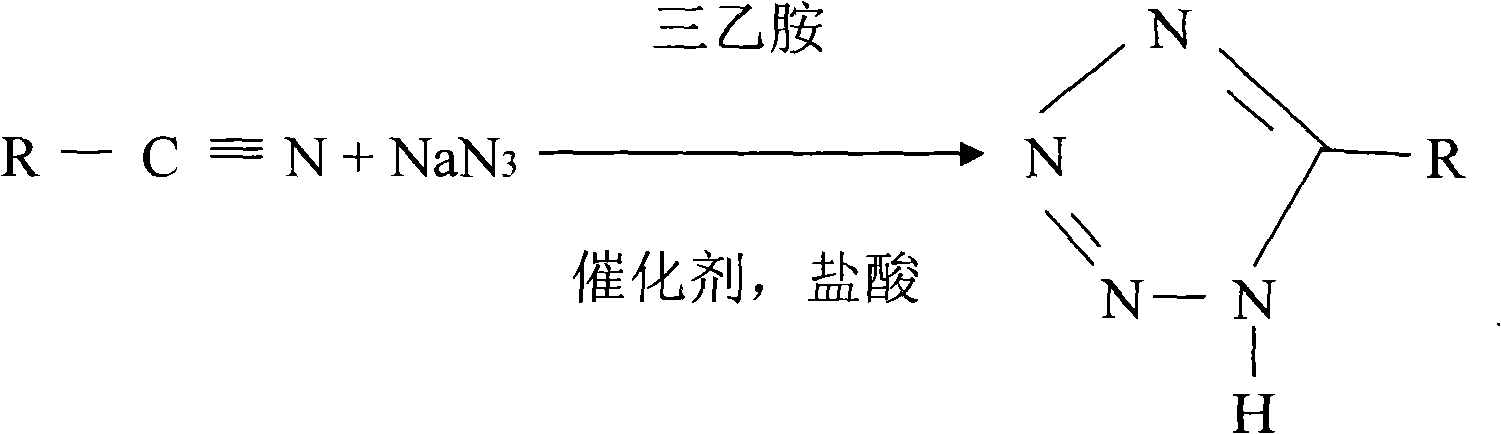
Production technique for preparing 5-alkyltetranitrozole by high pressure method
The invention discloses a production process using a high pressure method to prepare 5-alkane tetrazoline. Alkyl nitrile, sodium azide, triethylamine and triethylamine hydrochloric acid are mixed according to a certain proportion and then are plunged into a reaction kettle to react to generate 5-alkane tetrazoline; two reaction equations exist due to the difference of the raw material alkyl nitrile. The production process is easy to operate, and has high product yield, no waste water and small pollution.
Owner:江苏德峰药业有限公司 +1
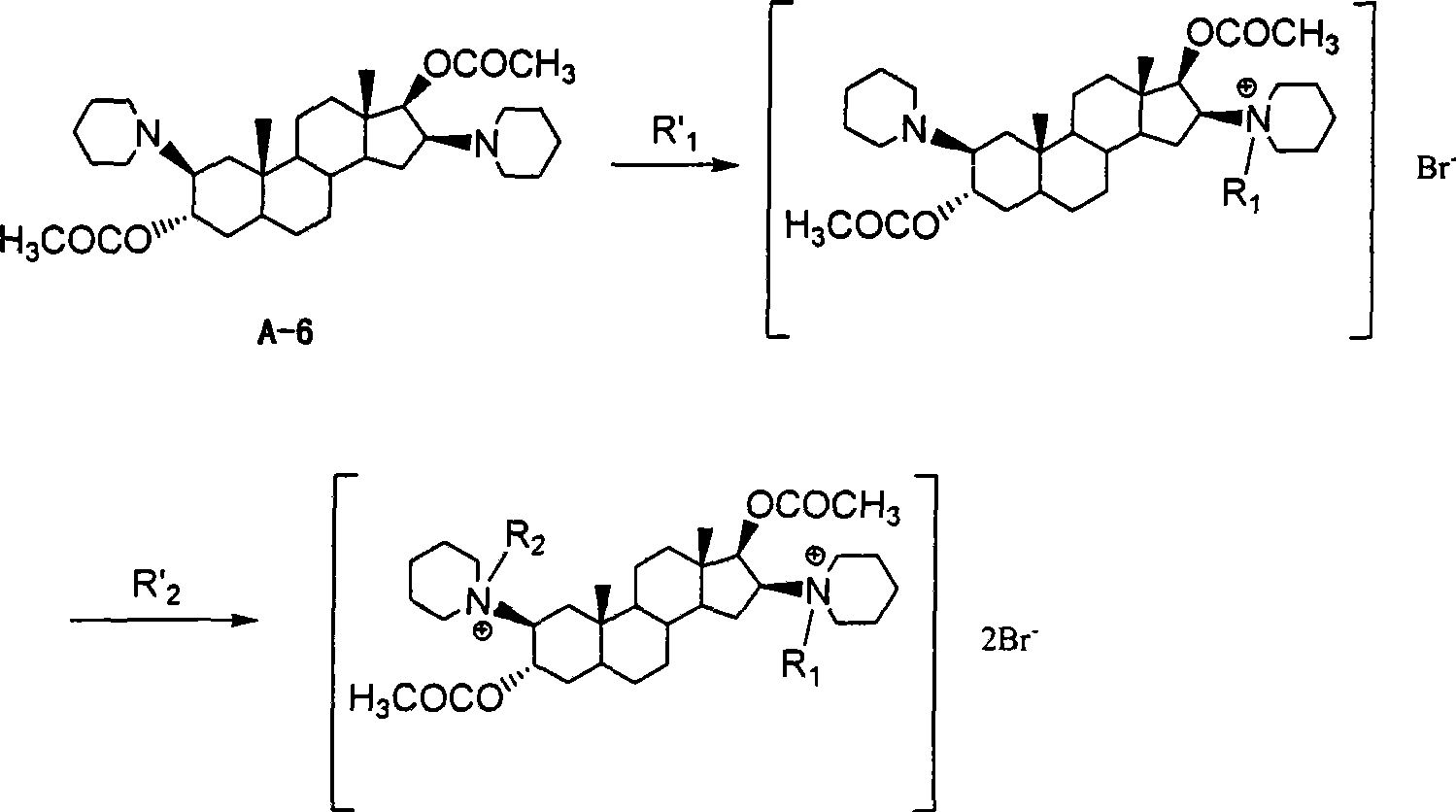
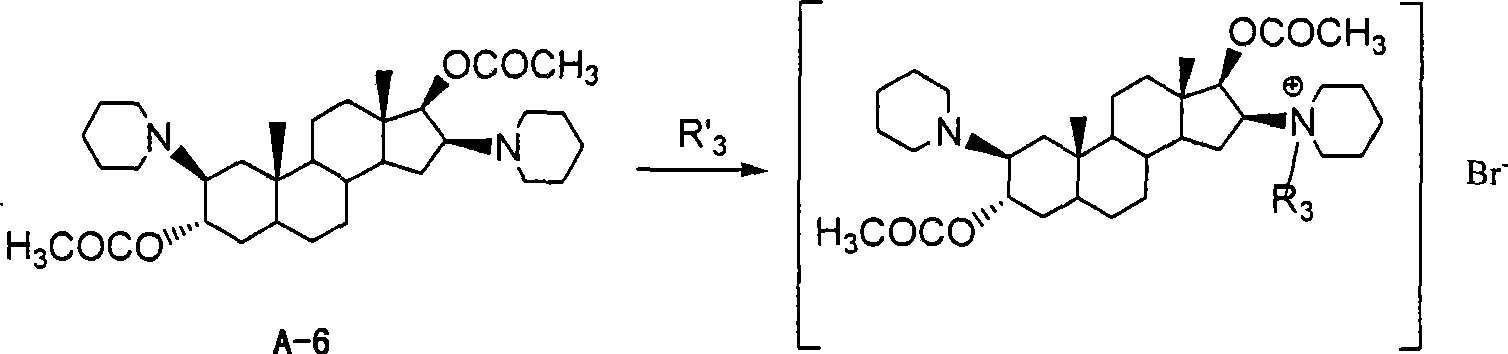
Bisamidocyanogen androstanes derivates, preparation method and use thereof
InactiveCN101392014AStrong muscle relaxant effectQuick effectOrganic active ingredientsMuscular disorderAndrostanesNitrile
The invention discloses a diamino etioallocholane derivative, a preparation method and the application thereof. The structure of the derivative is shown as the formula (I) or the formula (II), wherein, R1 or R2 is respectively C1-4 alkyl, C1-4 nitrile group or C1-4 alkynyl independently; R3 is C2-4 alkyl, C1-4 nitrile group or C1-4 alkynyl. Through the determination of activity, the compound has stronger muscle-relaxing action, and the holding time of one-time intravenous thereof is short.
Owner:NANJING MESE PHARMA
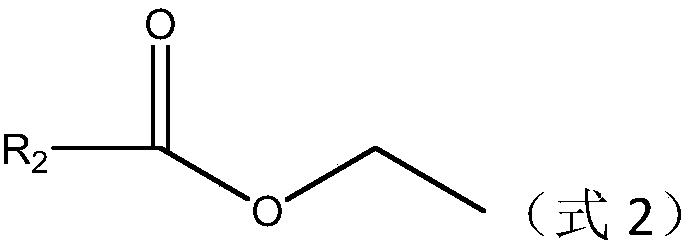
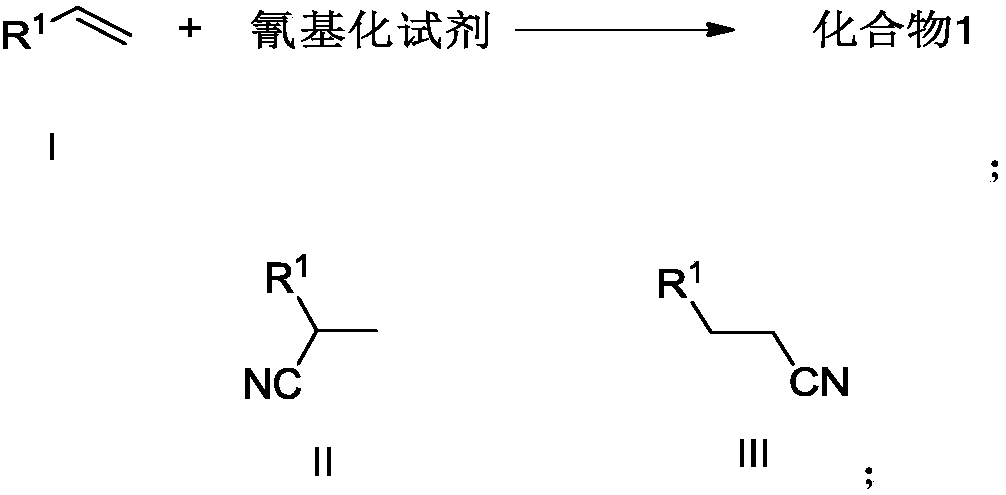
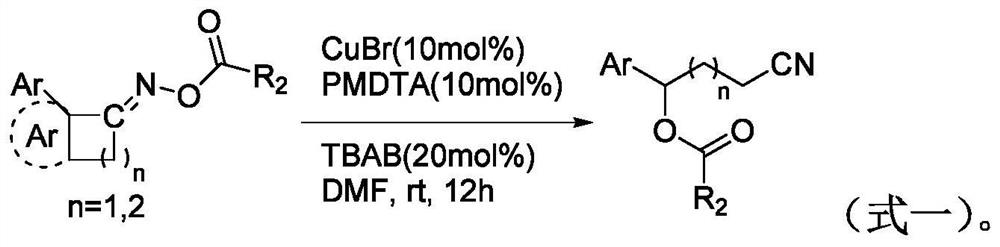
Preparation method of alkyl nitrile compound
ActiveCN111484472AReact efficientlyImplement responseGroup 4/14 element organic compoundsOrganic compound preparationOrganosolvCombinatorial chemistry
The invention discloses a preparation method of an alkyl nitrile compound. Specifically, the preparation method comprises the following step: in an organic solvent, in the presence of a protective gasand under the action of a catalyst, carrying out a reduction reaction as shown in the specification on olefin as shown in a formula I, a cyanation reagent and water, wherein the alkyl nitrile compound 1 is a compound II and / or a compound III. The preparation method provided by the invention is mild in condition, can realize hydrocyanation of olefin more safely and efficiently, and has good substrate universality and functional group compatibility.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Method for synthesizing cyanoalkyl indoline by cyano-alkylation of N-allylaniline
ActiveCN109824573AWide range of substratesMild reaction conditionsOrganic chemistryBulk chemical productionAlkyl transferIndoline
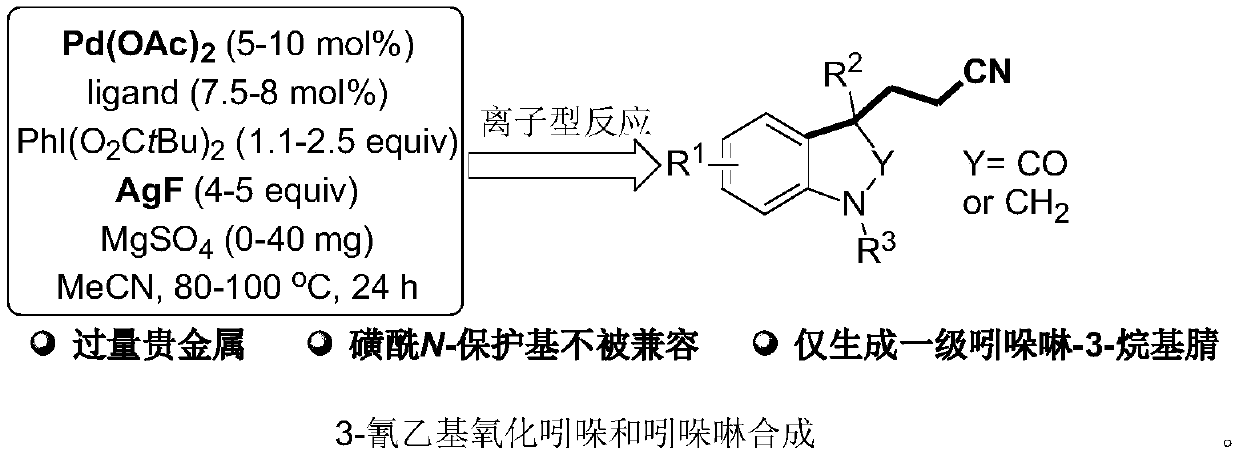
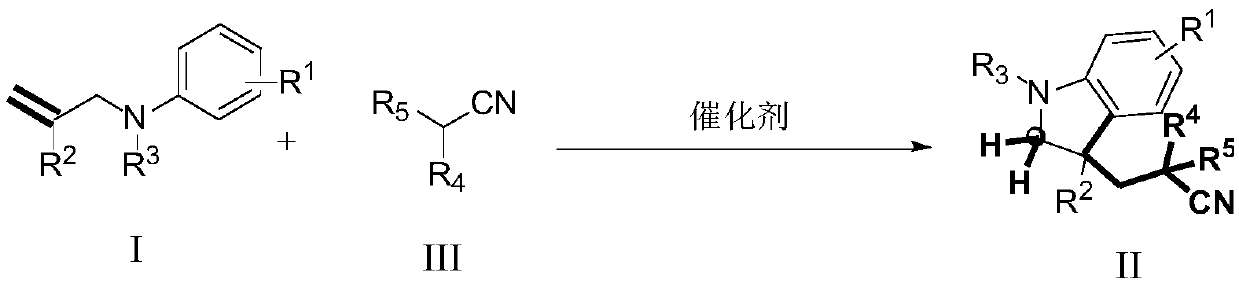
The invention provides a method for synthesizing cyanoalkyl indoline by cyano-alkylation of N-allylaniline. The method includes using cheap alkyl nitrile and specific non-activated olefin to perform an exo selective cyano-alkylation / cyclization cascade reaction on the N-allylaniline compound under the conditions of no metal and neutrality to generate 3-cyanoalkyl indoline. The reaction of the method does not need metal and strong alkali, has a wide substrate range, and can smoothly occur even when sulfonyl protecting groups exist in the compound; and in addition, by using the method, primary,secondary and tertiary nitrile products can be successfully synthesized, and meanwhile, the reaction conditions are mild, the operation is simple, so that the method is suitable for industrial popularization.
Owner:KUNMING UNIV
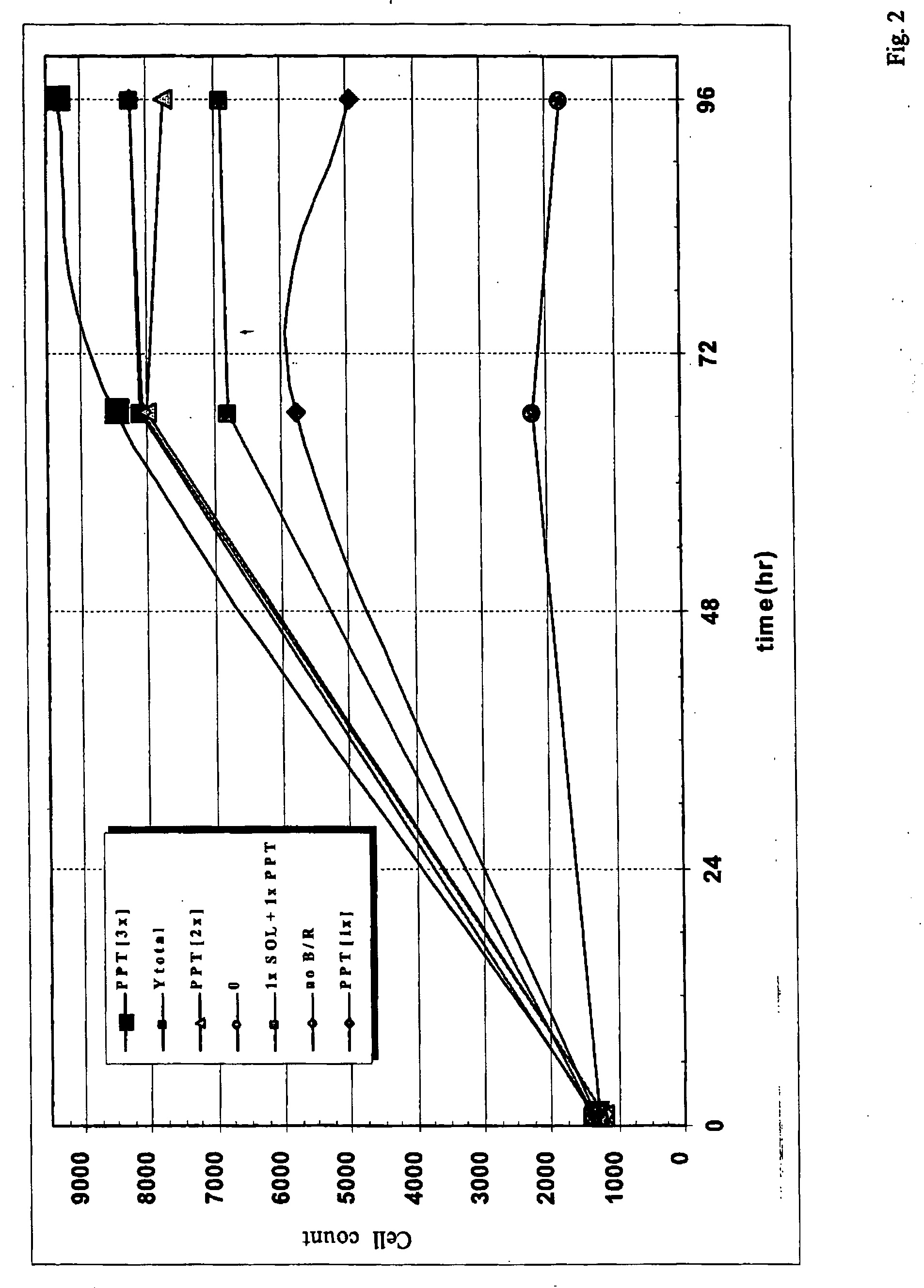
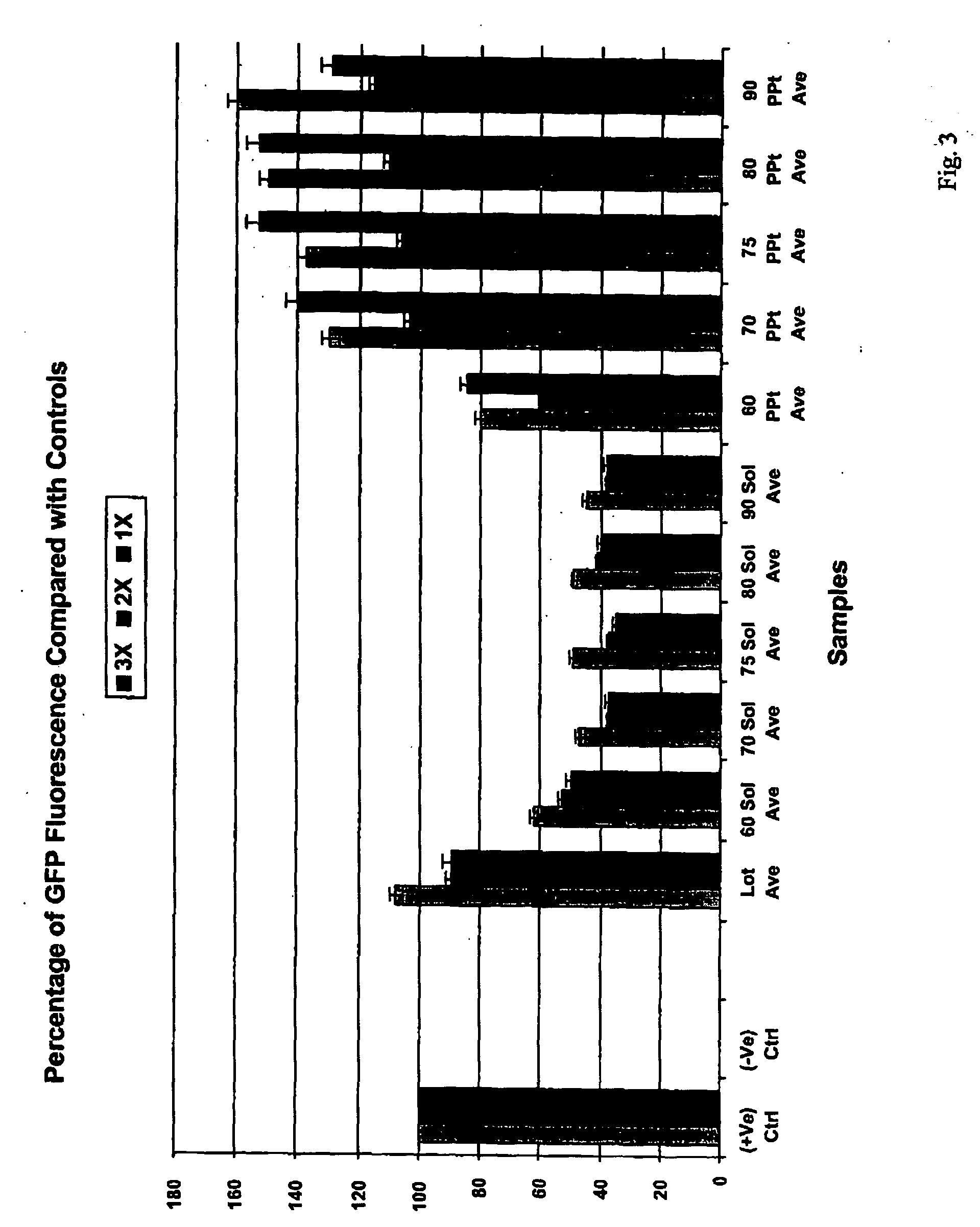
Preparation, characterization and quantification of active fractions that promote cell growth and recombinant-protein expression
InactiveUS20050250177A1High activityPromote growthCulture processCell culture mediaHydrolysateKetone
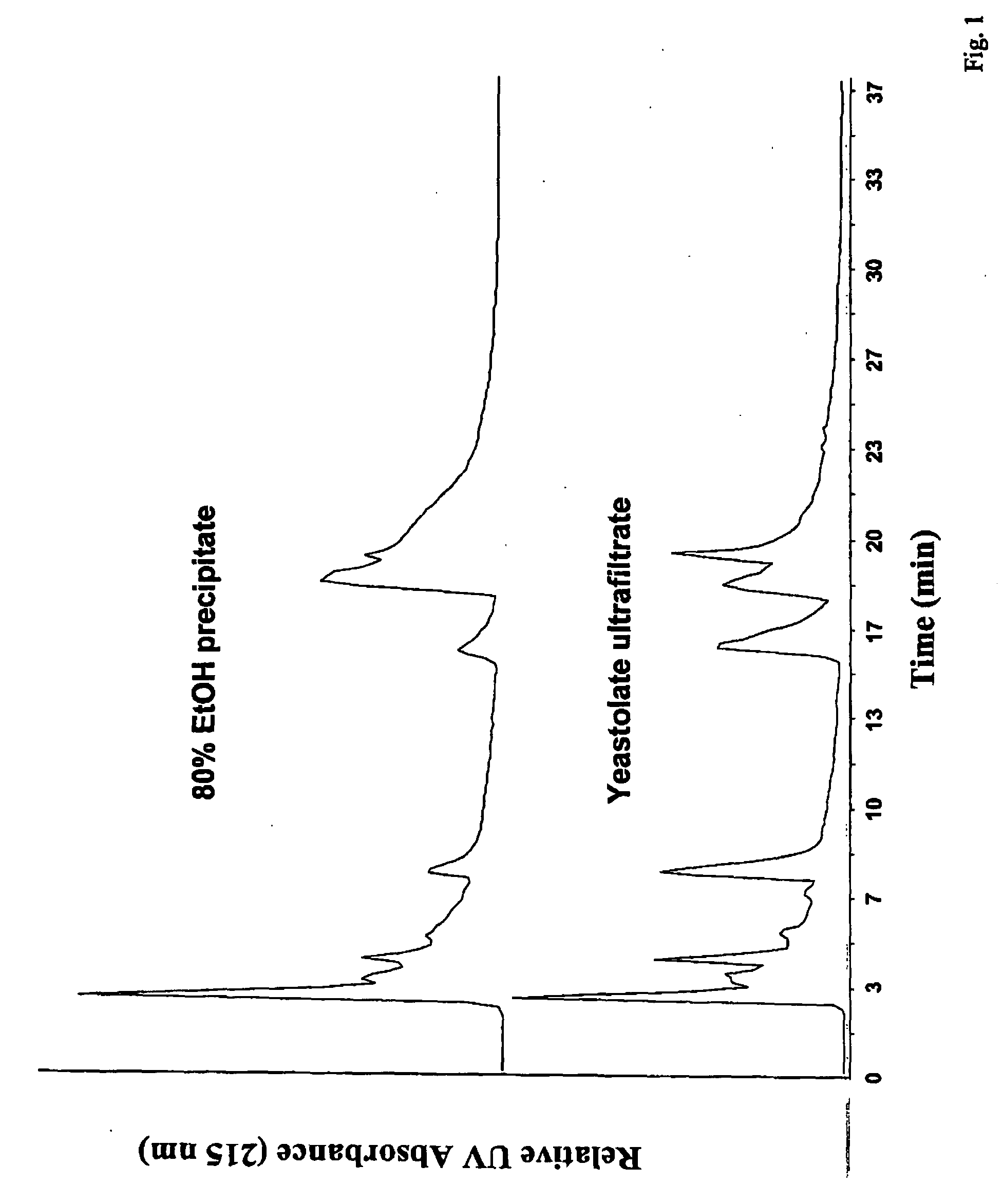
A process for preparing an active animal cell-growth-enhancing fraction of a hydrolysate of plant tissue, animal tissue or microorganism ultrafiltrate material, especially a fraction of a yeastolate ultrafiltrate, comprising forming a precipitate from said fraction with a water-soluble solvent is disclosed. Such solvents as alkanols, alkyl sulfoxides, ketones or alkyl nitriles, especially lower (C1-C5) alkanols, particularly ethanol, can be used. The invention also relates to an active animal cell-growth-enhancing fraction of a hydrolysate of plant tissue, animal tissue or microorganism ultrafiltrate material, especially a fraction of a yeastolate ultrafiltrate, substantially free of aromatic and methyl group bearing compounds which has improved cell-growth-enhancing properties.
Owner:BIOPROCESSING TECH CENT +2
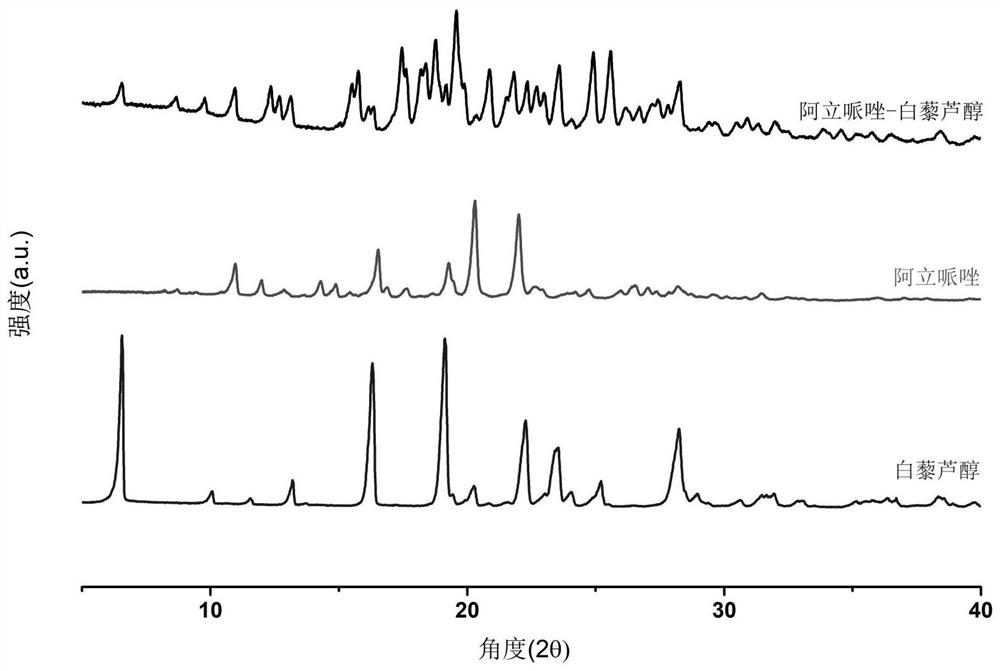
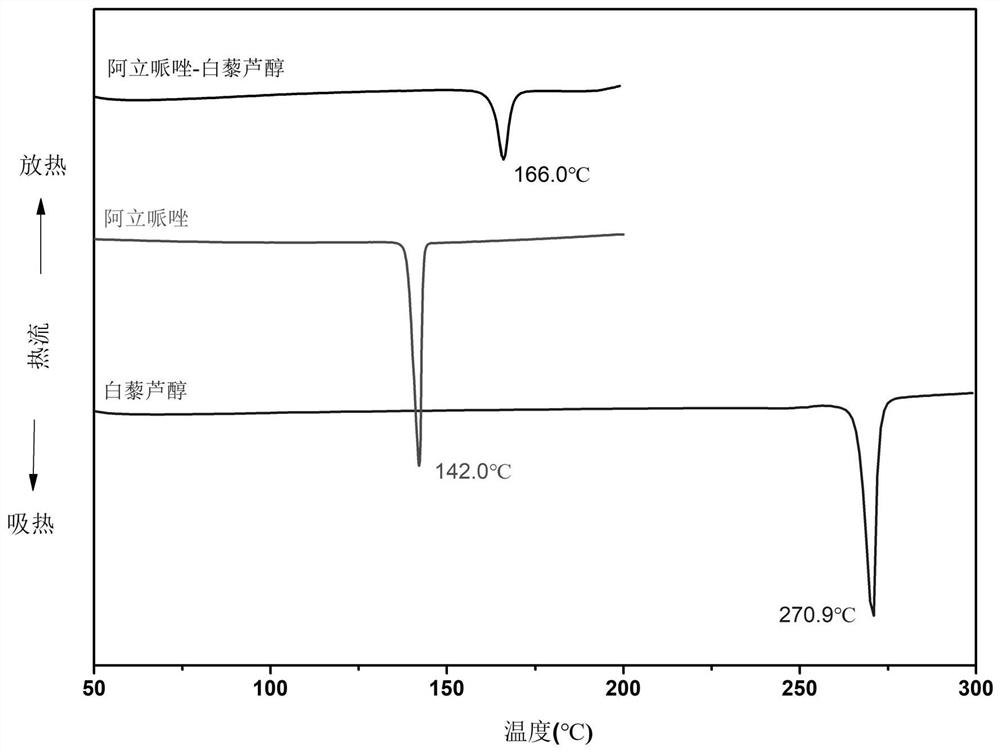
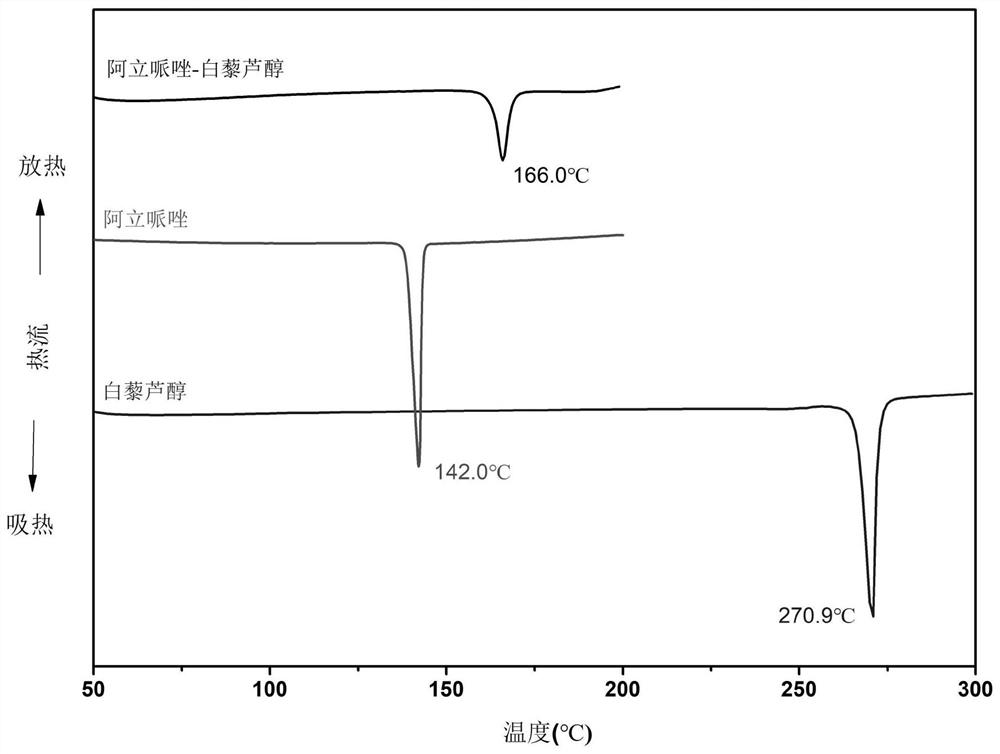
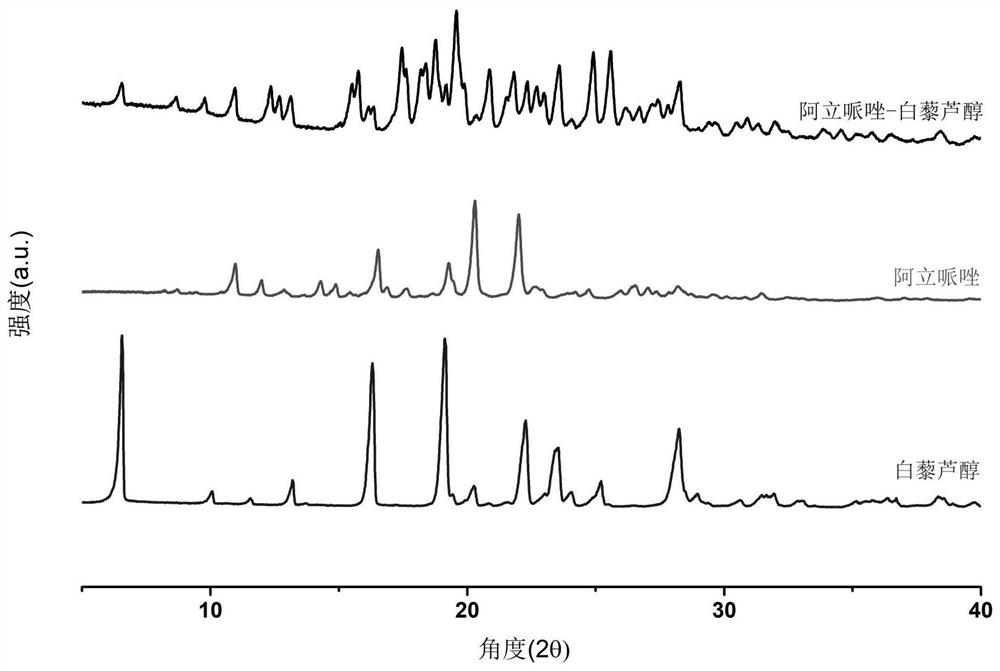
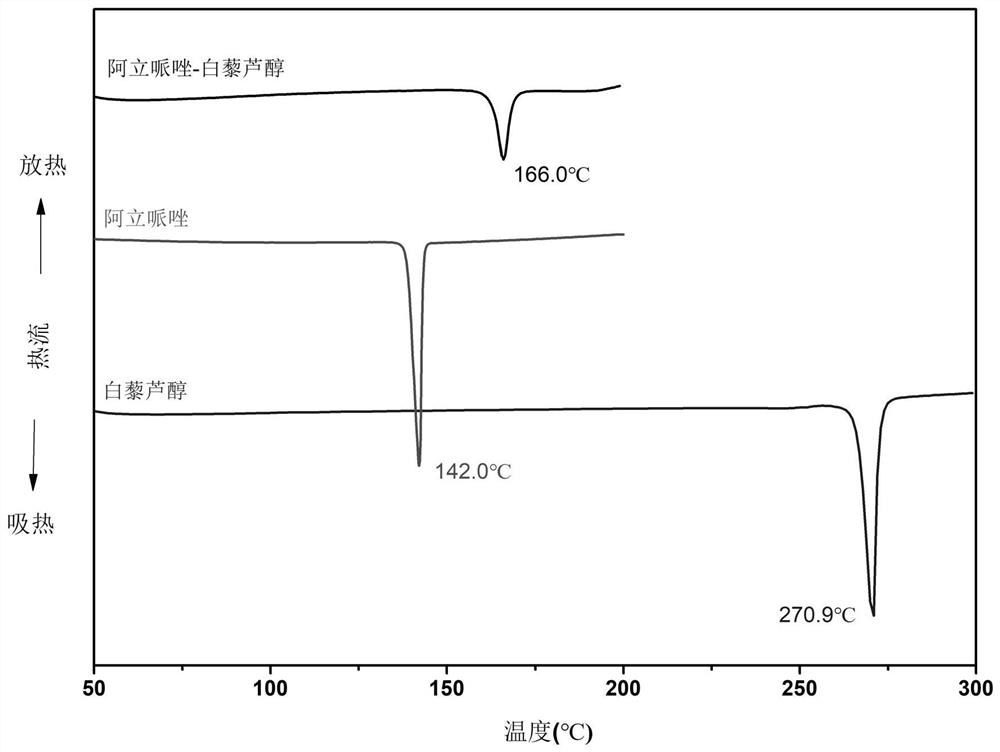
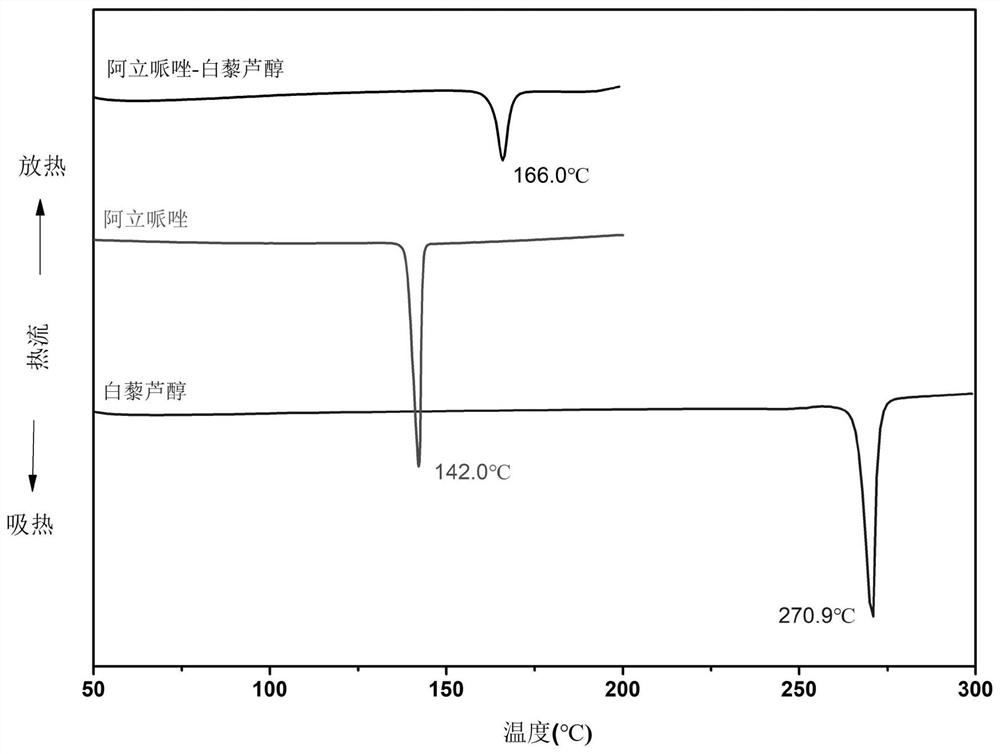
Aripiprazole pharmaceutical co-crystal and preparation method thereof
ActiveCN113105389AMaintain pharmacological activityReduce solubilityNervous disorderOrganic chemistryAcetic acidO-Phosphoric Acid
The invention discloses an aripiprazole pharmaceutical co-crystal and a preparation method thereof. The aripiprazole pharmaceutical co-crystal is formed through intermolecular hydrogen bonds by taking aripiprazole as an active pharmaceutical ingredient and resveratrol as a precursor. The preparation method of the aripiprazole pharmaceutical co-crystal comprises the following steps: grinding aripiprazole and resveratrol in a mortar with equal mole, adding a ketone or alkyl nitrile solvent before grinding, manually grinding for 30 minutes, and drying in vacuum to obtain the aripiprazole pharmaceutical co-crystal. A powder dissolution experiment of aripiprazole and resveratrol shows that the dissolution rate of aripiprazole is slowed down in an acetic acid buffer solution with the pH value of 4.0 and a phosphoric acid buffer solution with the pH value of 6.8, and a potential slow release effect is shown, so that the muscle injection prepared from the aripiprazole eutectic crystal can be used for treating schizophrenia in a long-acting manner, can be used for preventing and treating the schizophrenia, and reduces the muscle irritation.
Owner:JIANGSU OCEAN UNIV
FPR1 antagonist derivatives and use thereof
A dipeptide derivative as formyl peptide receptor 1 (FPR1) antagonist is provided. The dipeptide derivative is represented by formula (I),wherein:the chiral centers in formula (I) are S and R configurations respectively;each of RK and RT is selected from a group consisting of a hydrogen, a hydroxyl group, a C1-C4 alkyl-substituted hydroxyl group, a C1-C4 alkoxyl group, a carboxylic acid group, a C1-C4 alkyl nitrile-substituted, C1-C4 alkyl-substituted or C1-C4 alkoxyl-substituted amido group, a C1-C4 alkyl-substituted ester group and a benzoyl group having a C1-C4 alkyl-substituted benzene ring; and each of RM and RS is selected from a group consisting of a hydrogen, a hydroxyl group, a phenyl group, a pyridinyl group, a carboxylic acid group, a C1-C4 alkoxyl substituted ester group, and a benzoyl group having a hydroxyl-substituted, a halogen-substituted, a C1-C4 alkoxyl-substituted or a C1-C4 alkyl-substituted benzene ring.
Owner:CHANG GUNG UNIVERSITY
Preparation method of alkyl nitrile compound
ActiveCN111099941AAchieve cyanationImprove compatibilityPreparation by cyanide reactionSteroidsNickel saltPtru catalyst
The invention discloses a preparation method of an alkyl nitrile compound shown as formula I. The preparation method comprises the following step: in a solvent, in the presence of an additive and a catalyst, Zn (CN) 2 and an alkyl halide shown as formula II are subjected to a coupling reaction as shown in the specification to obtain the alkyl nitrile compound as shown in the formula I, wherein theadditive comprises an alkali, the catalyst comprises a nickel compound and a phosphine ligand; the nickel compound is one or more of zero-valent nickel, monovalent nickel salt and divalent nickel salt; when the nickel compound contains zero-valent nickel or divalent nickel salt, the catalyst further comprises a reducing agent. According to the preparation method disclosed by the invention, cyanation of an alkyl halide can be simply, conveniently and efficiently realized by using a cheap catalytic system, and the preparation method also has good functional group compatibility and substrate universality.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
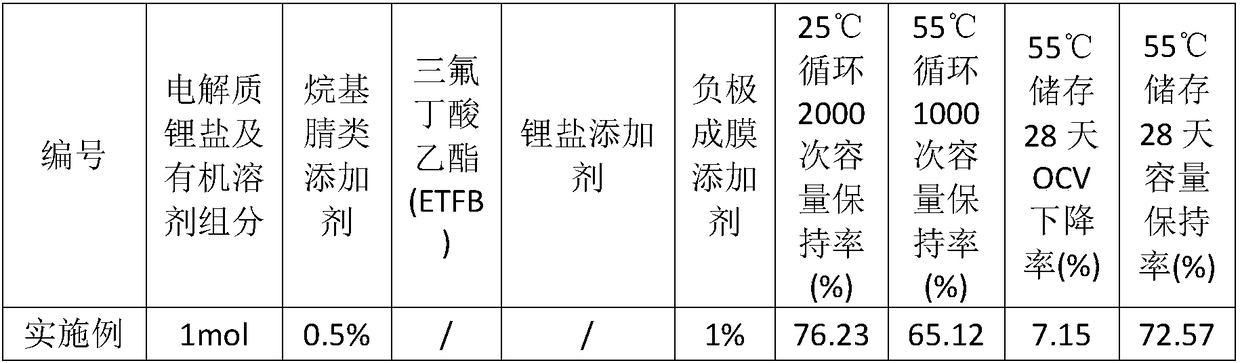
Lithium ion battery electrolyte additive for high-nickel system, electrolyte and battery
ActiveCN111244550ALower internal resistanceInhibit side effectsSecondary cells servicing/maintenanceOrganic electrolytesElectrolytic agentInternal resistance
Owner:TIANJIN ENERGIES
High-pressure production method of 5-hydroxyl alkyl tetrazole
The invention discloses a high-pressure production method of 5-hydroxyl alkyl tetrazole; and hydroxy alkyl nitrile, sodium azide and triethylamine react to prepare the 5-hydroxyl alkyl tetrazole under a high-temperature condition. The high-pressure production method of 5-hydroxyl alkyl tetrazole has simple process, is easy to operate, does not product wastewater and waste gas, and the product yield is high and reaches 85 percent.
Owner:NANTONG HUAFENG CHEM
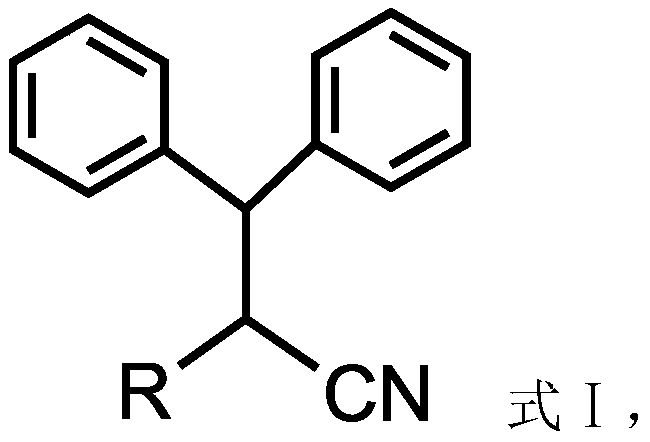
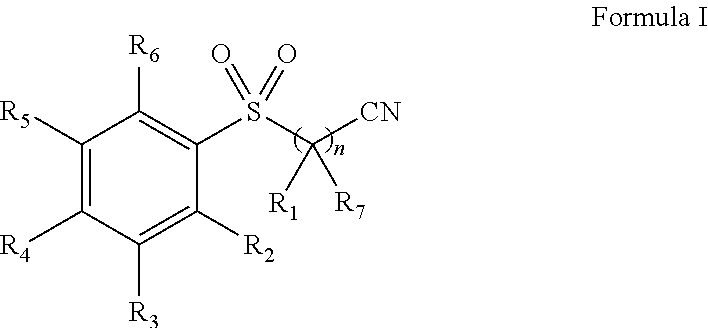
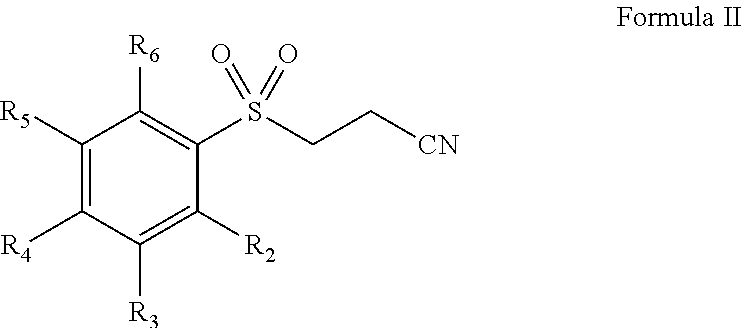
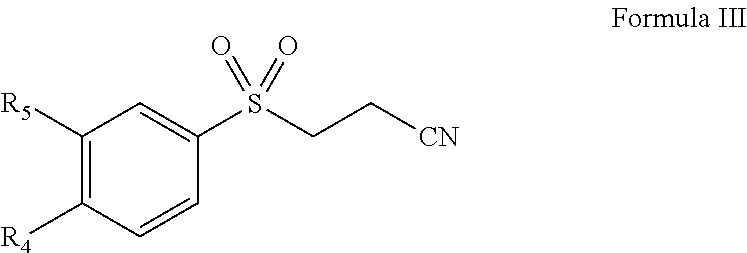
Pharmaceutical composition comprising omega-(arylsulfonyl)alkylnitrile
The present invention is directed to a pharmaceutical composition comprising a pharmaceutically acceptable carrier and a ω-(arylsulfonyl)alkylnitrile compound, or a pharmaceutically acceptable salt thereof. The present invention is also directed to a method for treating inflammation, inflammatory-related disorders, or pain, by administering an ω-(arylsulfonyl)alkylnitrile compound or a pharmaceutically acceptable salt or solvate thereof to a subject in need thereof.
Owner:OLATEC THERAPEUTICS INC
Preparation method of alkyl nitrile compound
InactiveCN111099942ASimple and efficient cyanationAchieve cyanationGroup 4/14 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsOrganic baseCombinatorial chemistry
The invention discloses a preparation method of an alkyl nitrile compound shown as formula I. The preparation method comprises the following step: in a solvent, in the presence of an additive, carrying out substitution reaction as shown in the specification on a cyanation reagent and an alkyl halide shown as formula II to obtain the alkyl nitrile compound shown as formula I, wherein the cyanationreagent is Zn (CN) 2 and / or Cu (CN) 2; the additive is one or more of an inorganic base, an organic base and a quaternary ammonium salt.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
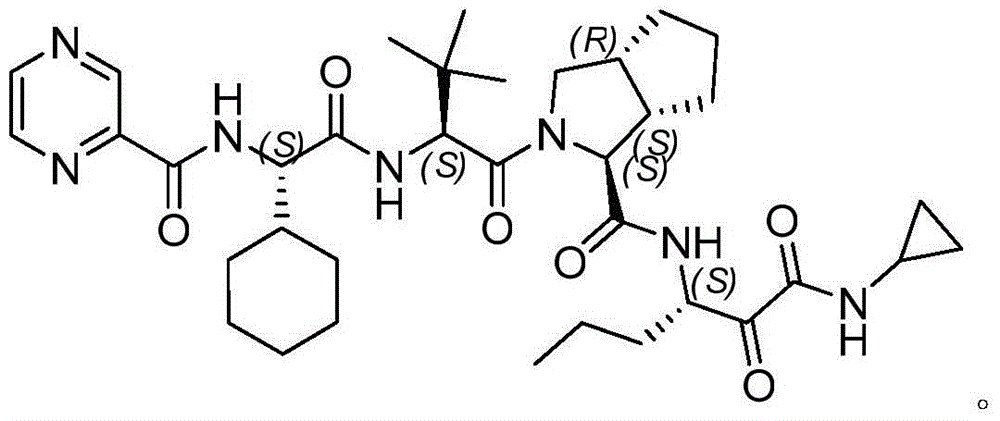
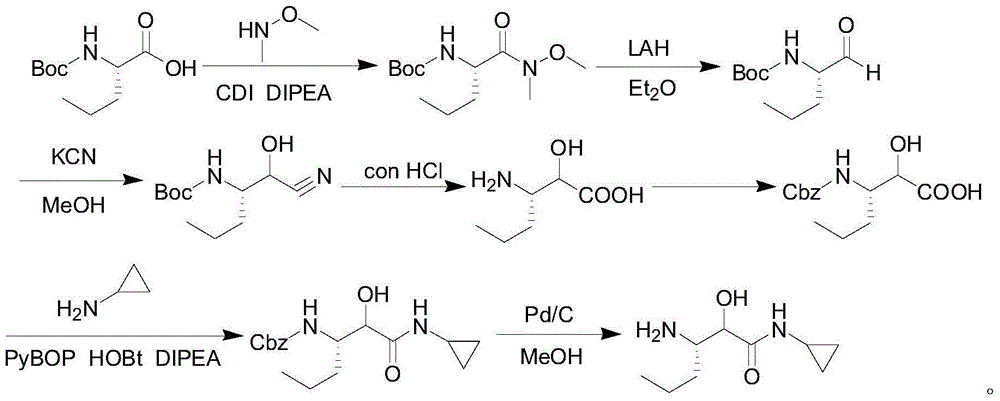
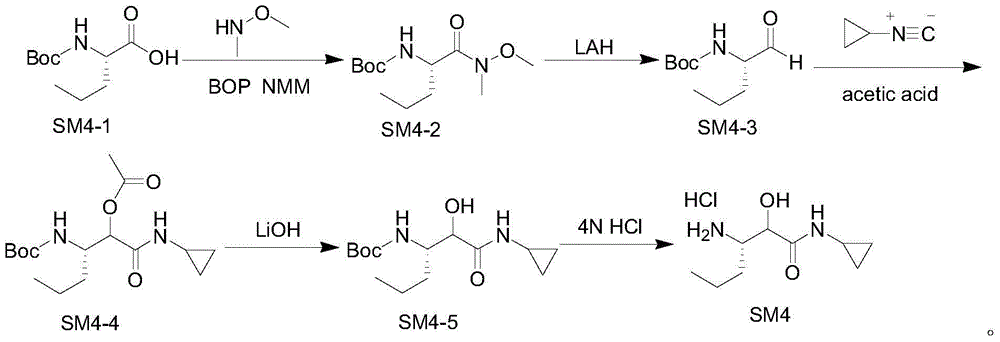
Intermediate for preparing N-cyclopropyl-(2S, 3S)-3-amino-2-hydroxy caproamide, preparation method and applications thereof
InactiveCN104860897ASimple preparation processHas industrial application valueCarbamic acid derivatives preparationOrganic compound preparationArylChemical structure
The present invention discloses an intermediate for preparing N-cyclopropyl-(2S, 3S)-3-amino-2-hydroxy caproamide, a preparation method and applications thereof, wherein the intermediate has a chemical structure general formula represented by a formula I, R is a C1-C4 alkyl or aryl substituted C1-C4 alkyl, and 3-propyl ethylene oxide-2-formic acid and alkyl nitrile or aryl nitrile react in the presence of a Lewis acid so as to prepare the intermediate. With application of the intermediate of the present invention to prepare N-cyclopropyl-(2S, 3S)-3-amino-2-hydroxy caproamide, advantages of high yield, simple process, no requirement of any toxic reagents and special equipment, cheap and easily-available raw materials and the like are provided, the chiral purity of the obtained target product is greater than 99%, and the method is suitable for large-scale production and has industrial application values.
Owner:SHANGHAI DESANO CHEM PHARMA +1
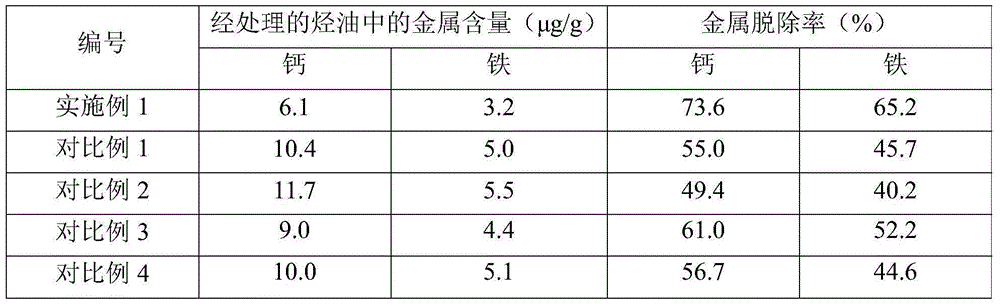
Composition used for reducing hydrocarbon oil metal content and its application and method for reducing hydrocarbon oil metal content
The invention discloses a composition used for reducing hydrocarbon oil metal content and an application thereof. The composition contains acid, a cyan-containing compound, aromatic hydrocarbon and a polyoxyethylene-polypropylene oxide segmented copolymer, the acid is selected from water-soluble phosphonic acid and water-soluble carboxylic acid, the polyoxyethylene-polypropylene oxide segmented copolymer takes phenolic resin or phenol-amine resin as a starting agent, and the cyan-containing compound is selected from aromatic nitrile with at least one hydrogen atoms substituted by C3-C8 branched-chain alkyls on aryl, and aralkyl nitrile with at least one hydrogen atom substituted by C3-C8 branched-chain alkyl on aryl. The invention also provides a method for reducing hydrocarbon oil metal content, which comprises mixing hydrocarbon oil with components in the composition and water, and a mixture is subjected to oil-water separation to obtain an oil phase and a water phase. The composition can effectively reduce the metal content of hydrocarbon oil, especially removes the calcium and iron in residuum.
Owner:CHINA PETROLEUM & CHEM CORP +1
A kind of aripiprazole medicine co-crystal and preparation method thereof
ActiveCN113105389BMaintain pharmacological activityReduce solubilityNervous disorderOrganic chemistryIntramuscular injectionEthylic acid
Owner:广西鲲宇药业有限公司
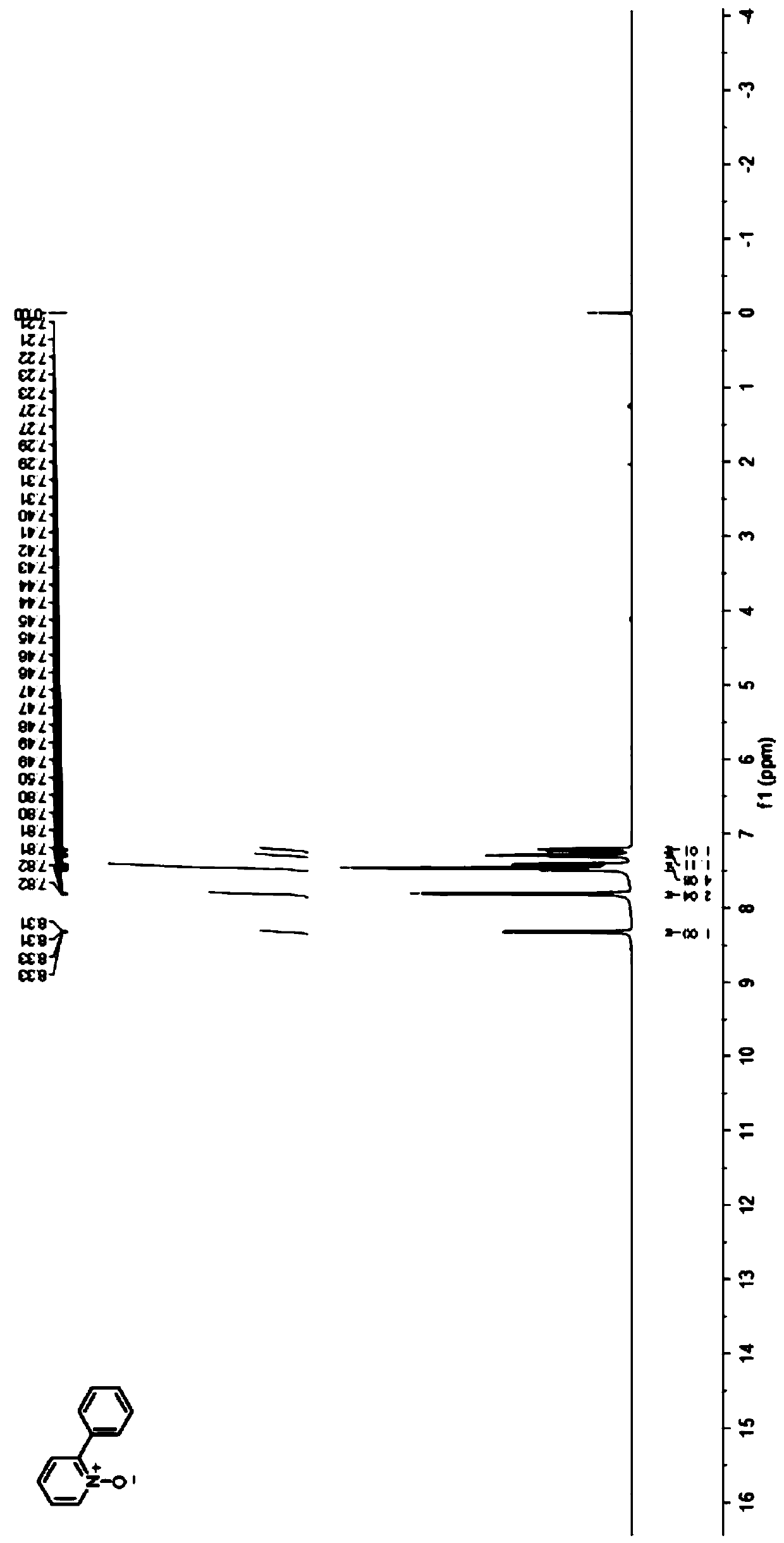
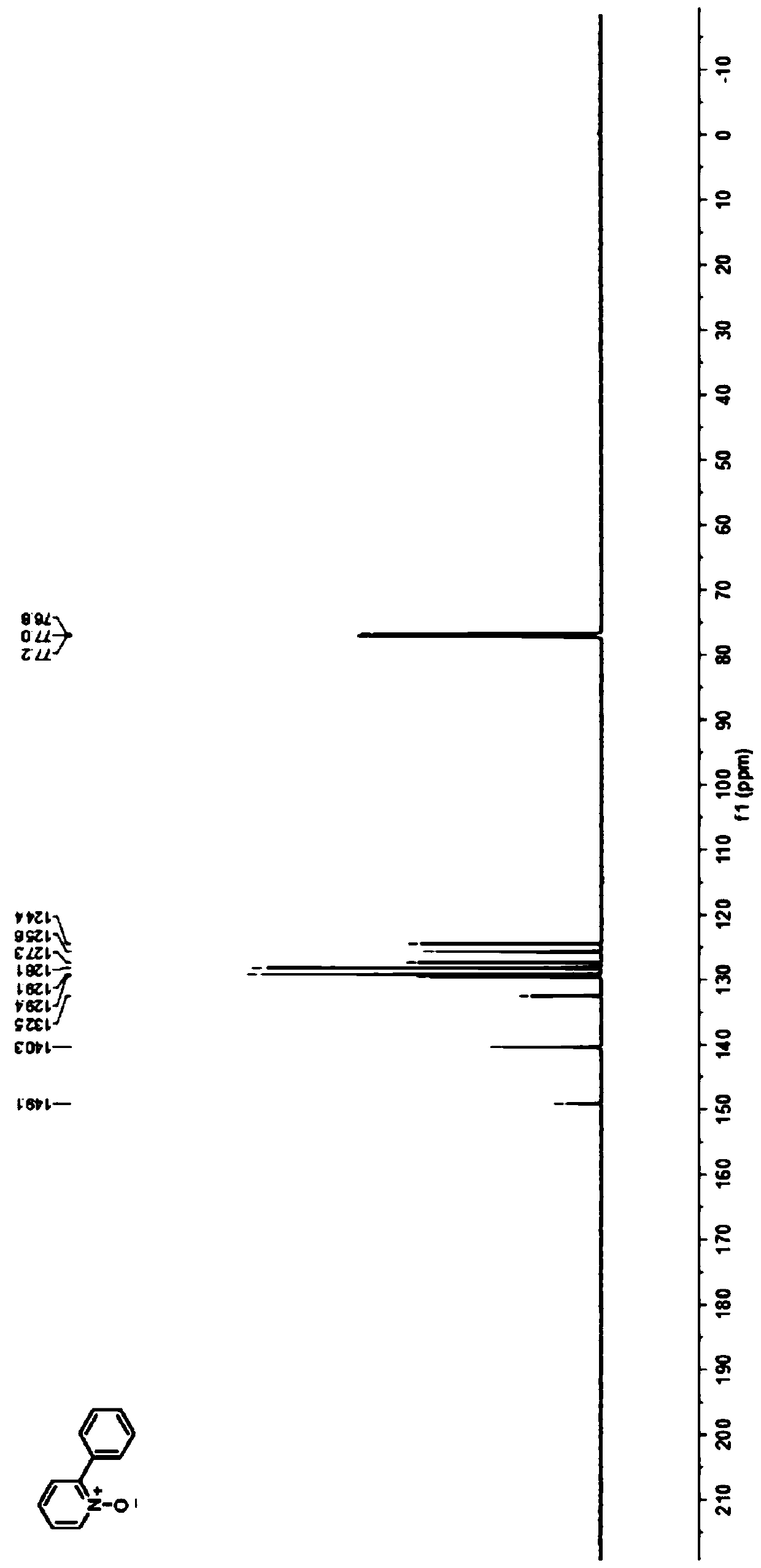
Preparation method of 2-phenyl substituted pyridine nitrogen oxide compound
ActiveCN110845404AReduce energy consumptionWith renewableOrganic chemistryTetrafluoroboratePtru catalyst
The invention discloses a preparation method of a 2-phenyl substituted pyridine nitrogen oxide compound, relates to a preparation method of a pyridine nitrogen oxide derivative, and aims to solve thetechnical problems of flammability, explosiveness, harsh reaction conditions and poorer regioselectivity of a catalyst in the conventional direct selective functionalization method. The method comprises the following steps: adding the pyridine nitrogen oxide derivative, diphenyl iodide tetrafluoroborate, an eosin Y catalyst, an alkali and an additive into a transparent reactor at room temperature,and sealing; replacing air in the reactor with nitrogen to form a nitrogen atmosphere, injecting a solvent, and uniformly mixing; irradiating the reactor with blue LEDs lamp light for reaction; afterthe reaction is finished, removing the solvent through rotary evaporation, and then performing separation and purification of prefabricated silica gel column chromatography to obtain the 2-phenyl substituted pyridine nitrogen oxide compound, and the structural formula of the 2-phenyl substituted pyridine nitrogen oxide compound is as shown in the specification, R1 is hydrogen, alkyl, nitrile group, halogen, phenyl or nitro; the 2-phenyl substituted pyridine nitrogen oxide compound can be used in the fields of screening of drug lead compounds or biological activity test and research.
Owner:HARBIN INST OF TECH
Flame retardant anti-bacterial composite modifier used in fiber field and preparation method thereof
InactiveCN102619086BFlame retardantImprove thermal stabilityGroup 5/15 element organic compoundsFibre treatmentBenzoic acidFiber
The invention relates to a flame retardant anti-bacterial composite modifier used in the fiber field and a preparation method thereof. The active components of the flame retardant anti-bacterial modifier are alkyl phosphate and alkyl guanidine; and the preparation method of the flame retardant anti-bacterial composite modifier comprises the following steps of: (1) preparing alkyl tetrol phosphoric acid ester; and (2) taking alkyl tetrol phosphate ester B, alkyl guanidine C and p-benzoic acid alkyl nitrile D which have the equal amount of substance, adding concentrated sulfuric acid and dicyclohexylcarbodiimide / N, N'-diisopropyl carbodiimide (DCC / DIC) as catalysts, wherein the mass ratio of the concentrated sulfuric acid to the alkyl tetrol phosphate ester B is 1:2-4, the mass ratio of DCC / DIC to the alkyl guanidine C is 1:2-5, rising the temperature to 70 to 200 DEG C, after reacting for 3-10h, cooling, filtering and drying to obtain the solid composite modifier E. The flame retardant anti-bacterial composite modifier has the advantages of having the flame retardant anti-bacterial performance, good thermal stability, being capable of meeting the high temperature processing requirement and being not easy to degrade; and products machined and prepared by using the composite modifier are not easy to oxidize and change color; and the flame retardant anti-bacterial composite modifier is widely applied.
Owner:ZHEJIANG JIABAO NEW FIBER GROUP
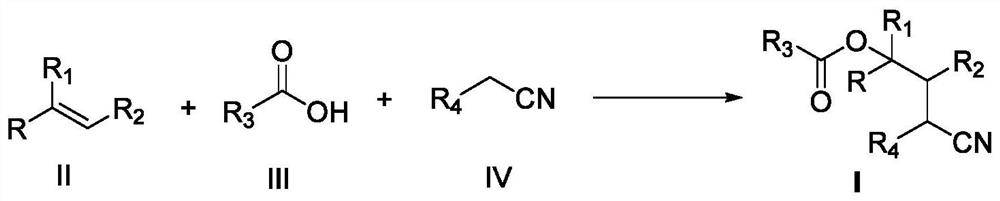
A kind of preparation method of gamma-carboxylated alkyl nitrile compound
ActiveCN109912456BConvenient sourceLow priceCarboxylic acid nitrile preparationOrganic compound preparationNickel catalystPolymer science
The invention discloses a preparation method of gamma-carboxylated alkyl nitrile compounds. The method uses an olefin compound of formula II, a carboxylic acid compound of formula III, and an alkyl nitrile compound of formula IV in a nickel catalyst, silver A series of γ-carboxylated alkyl nitrile compounds with various structures were prepared by carrying out the difunctionalization reaction of olefins under the condition of oxidizing agent. The synthesis route of the gamma-carboxylated alkyl nitrile compound of the present invention has the advantages of easy source of raw materials, low price of the catalytic system, wide application range of reaction substrates and high yield of target products.
Owner:NANCHANG HANGKONG UNIVERSITY
A gastrodin compound and its preparation method, preparation and application
ActiveCN107056853BImprove bioavailabilityGood curative effectOrganic active ingredientsUrea derivatives preparationDiseaseCyclic ether
The invention discloses a gastrodin compound and a preparation method, preparation and application thereof. The gastrodin compound is co-crystal of a compound as shown in the formula (I ) and urea, and the structural formula of the compound of the formula (I) is as shown in the specification. According to the preparation method, gastrodin and the urea react with each other in an organic solvent system to prepare the gastrodin compound, and the organic solvent is one or more of alcohols, ketones, alkyl nitrile and cyclic ethers. The preparation is powder, granules, tablets, capsules and pills which are prepared by adding pharmaceutically acceptable adjuvant materials in the gastrodin compound. The application is application of the gastrodin compound in preparation of drugs for preparing drugs for preventing and treating cardiovascular and cerebrovascular diseases. The solubility of the co-crystal of the gastrodin and the urea is high, the medicine absorbing efficiency is improved, the bioavailability of the medicine is improved, and therefore, the gastrodin compound plays an important role in improvement of curative effect of the medicine and the safety.
Owner:KPC PHARM INC +1
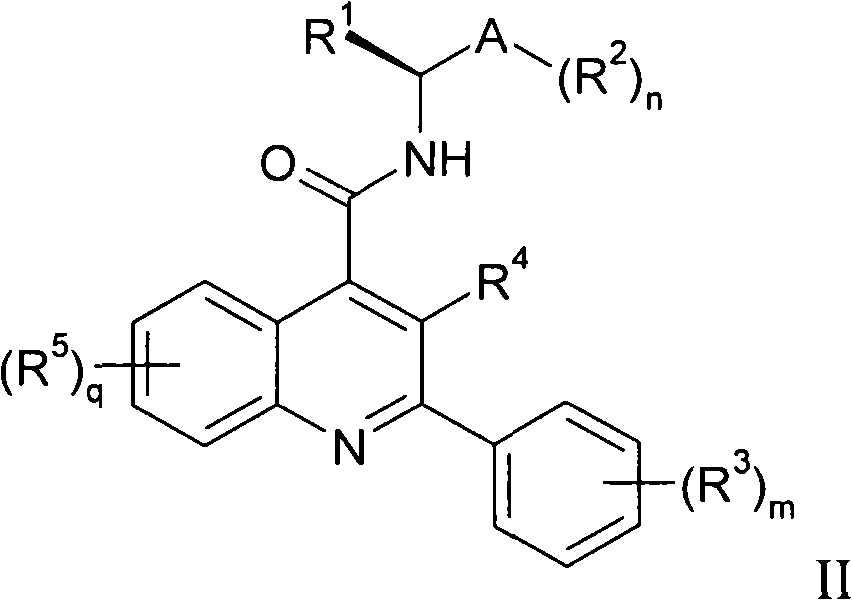
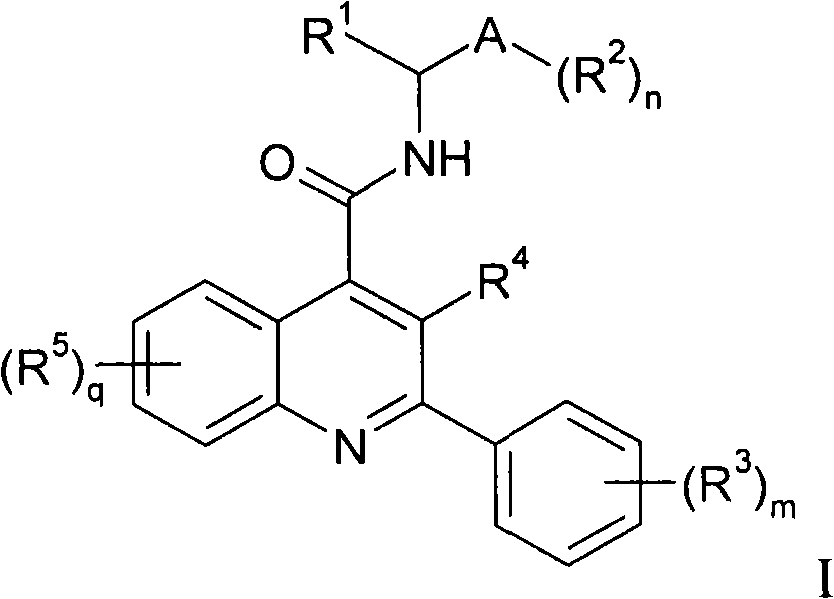
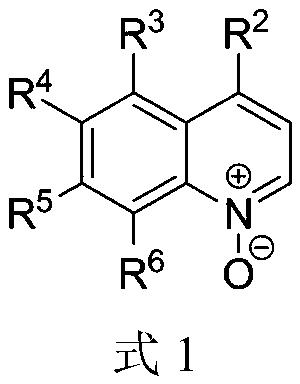
Alkylnitrile quinolines, as NK-3 receptor ligands
InactiveCN101268053AImprove solubilityPromote absorptionNervous disorderOrganic chemistryReceptorQuinoline
Owner:ASTRAZENECA AB
A kind of preparation method of ultrasonic-assisted n-(quinolin-2-yl) alkyl amides
InactiveCN108409652BAbundant and easy to obtainSolve pollutionOrganic chemistryQuinoloneAcid catalysis
The invention discloses an ultrasound-assisted preparation method of N-(quinoline-2-yl) alkyl amide. Under ultrasound assistance and catalysis of sulfonic acid cationic exchange resin, C2-N-amidationis performed on quinolone N-oxide, so as to obtain N-(quinoline-2-yl) alkyl amide. The method uses raw materials easy to obtain, is performed under simple, mild reaction conditions, is environmentallyfriendly and energy-saving, and has high reaction selectivity and yield; substrate functional groups are highly compatible; particularly, the sulfonic acid cationic exchange resin is used as an acidcatalyst, is easy to recycle and reuse, lowers reaction costs, and solves the pollution problem of traditional proton acid catalysis reaction, thereby having higher application value.
Owner:CENT SOUTH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com